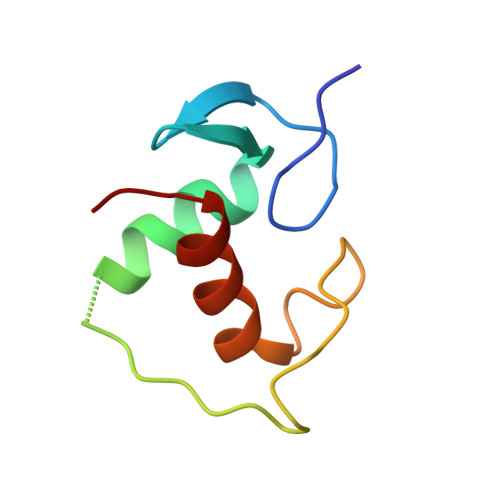
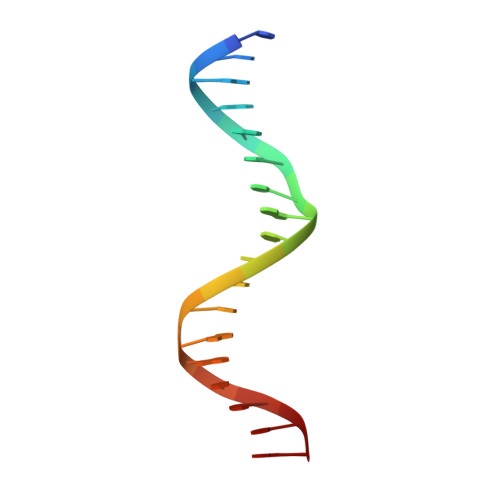
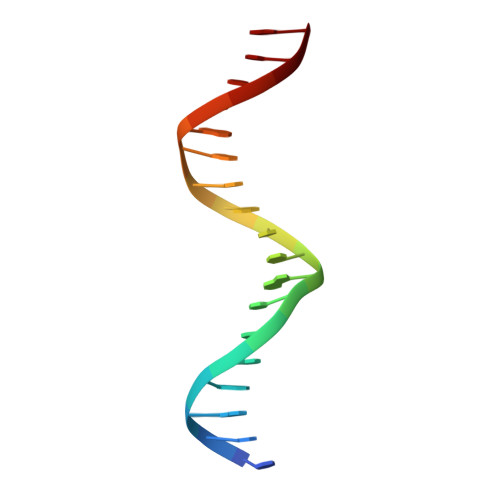
The oestrogen receptor recognizes an imperfectly palindromic response element through an alternative side-chain conformation.
Schwabe, J.W., Chapman, L., Rhodes, D.(1995) Structure 3: 201-213
- PubMed: 7735836
- DOI: https://doi.org/10.1016/s0969-2126(01)00150-2
- Primary Citation of Related Structures:
4AA6 - PubMed Abstract:
Structural studies of protein-DNA complexes have tended to give the impression that DNA recognition requires a unique molecular interface. However, many proteins recognize DNA targets that differ from what is thought to be their ideal target sequence. The steroid hormone receptors illustrate this problem in recognition rather well, since consensus DNA targets are rare. Here we describe the structure, at 2.6 A resolution, of a complex between a dimer of the DNA-binding domain from the human oestrogen receptor (ERDBD) and a non-consensus DNA target site in which there is a single base substitution in one half of the palindromic binding site. This substitution results in a 10-fold increase in the dissociation constant of the ERDBD-DNA complex. Comparison of this structure with a structure containing a consensus DNA-binding site determined previously, shows that recognition of the non-consensus sequence is achieved by the rearrangement of a lysine side chain so as to make an alternative base contact. This study suggests that proteins adapt to recognize different DNA sequences by rearranging side chains at the protein-DNA interface so as to form alternative patterns of intermolecular contacts.
Organizational Affiliation:
Medical Research Council, Laboratory of Molecular Biology, Cambridge, UK.