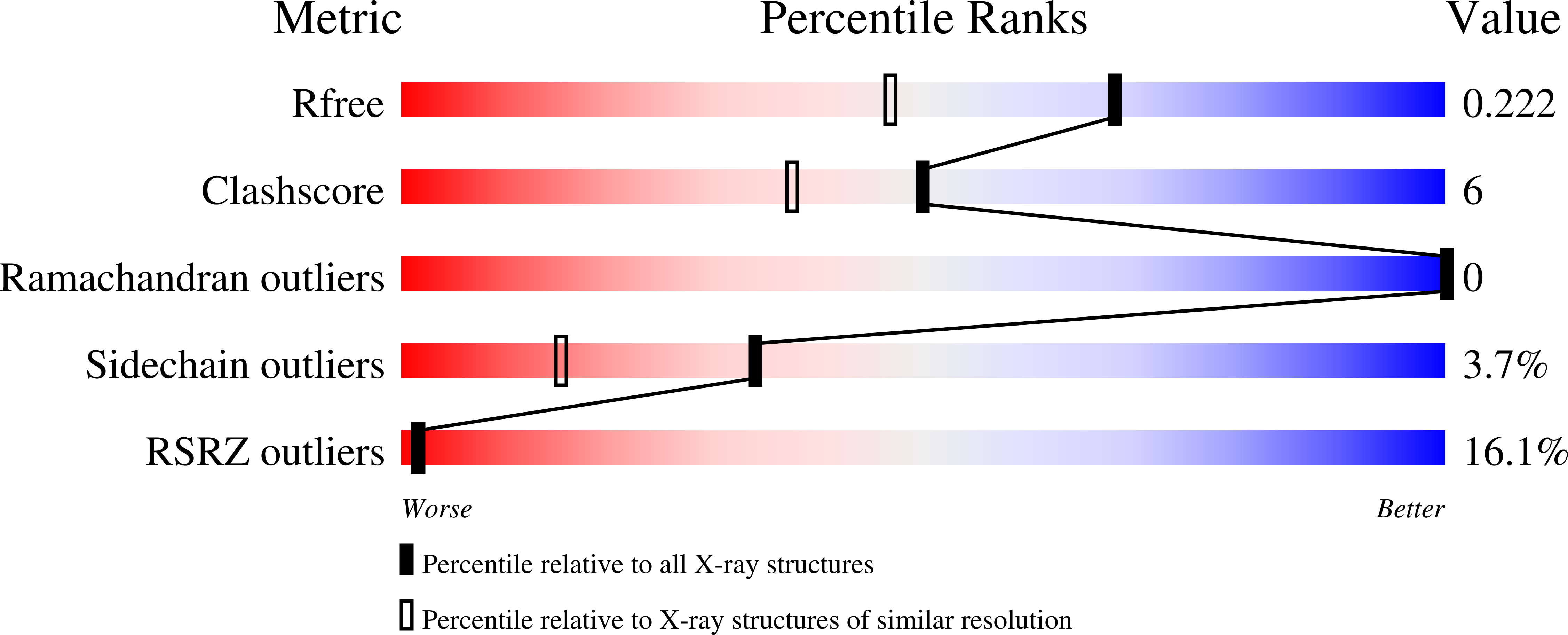
Crystal structure of the minimalist max-e47 protein chimera.
Ahmadpour, F., Ghirlando, R., De Jong, A.T., Gloyd, M., Shin, J.A., Guarne, A.(2012) PLoS One 7: e32136-e32136
- PubMed: 22389683
- DOI: https://doi.org/10.1371/journal.pone.0032136
- Primary Citation of Related Structures:
3U5V - PubMed Abstract:
Max-E47 is a protein chimera generated from the fusion of the DNA-binding basic region of Max and the dimerization region of E47, both members of the basic region/helix-loop-helix (bHLH) superfamily of transcription factors. Like native Max, Max-E47 binds with high affinity and specificity to the E-box site, 5'-CACGTG, both in vivo and in vitro. We have determined the crystal structure of Max-E47 at 1.7 Å resolution, and found that it associates to form a well-structured dimer even in the absence of its cognate DNA. Analytical ultracentrifugation confirms that Max-E47 is dimeric even at low micromolar concentrations, indicating that the Max-E47 dimer is stable in the absence of DNA. Circular dichroism analysis demonstrates that both non-specific DNA and the E-box site induce similar levels of helical secondary structure in Max-E47. These results suggest that Max-E47 may bind to the E-box following the two-step mechanism proposed for other bHLH proteins. In this mechanism, a rapid step where protein binds to DNA without sequence specificity is followed by a slow step where specific protein:DNA interactions are fine-tuned, leading to sequence-specific recognition. Collectively, these results show that the designed Max-E47 protein chimera behaves both structurally and functionally like its native counterparts.
Organizational Affiliation:
Department of Biochemistry and Biomedical Sciences, McMaster University, Hamilton, Ontario, Canada.