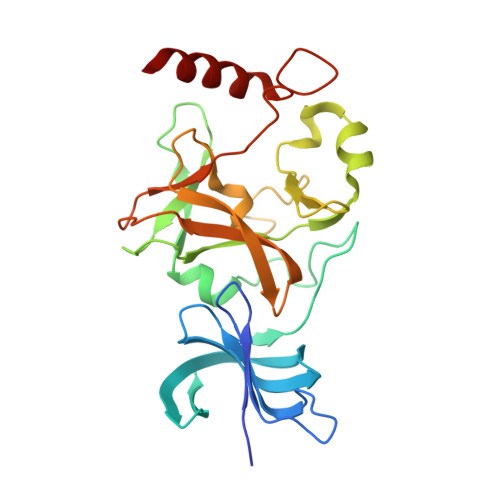
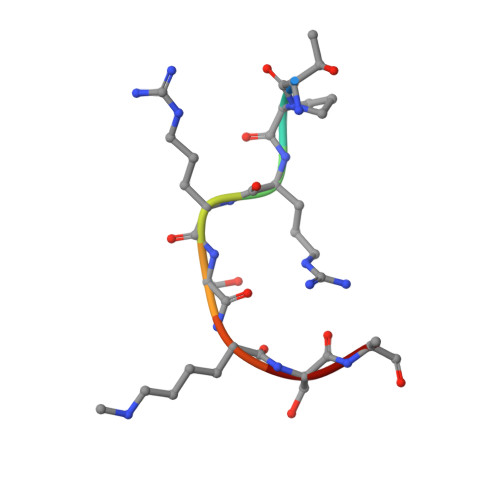
A methylation and phosphorylation switch between an adjacent lysine and serine determines human DNMT1 stability.
Esteve, P.O., Chang, Y., Samaranayake, M., Upadhyay, A.K., Horton, J.R., Feehery, G.R., Cheng, X., Pradhan, S.(2011) Nat Struct Mol Biol 18: 42-48
- PubMed: 21151116
- DOI: https://doi.org/10.1038/nsmb.1939
- Primary Citation of Related Structures:
3OS5 - PubMed Abstract:
The protein lysine methyltransferase SET7 regulates DNA methyltransferase-1 (DNMT1) activity in mammalian cells by promoting degradation of DNMT1 and thus allows epigenetic changes via DNA demethylation. Here we reveal an interplay between monomethylation of DNMT1 Lys142 by SET7 and phosphorylation of DNMT1 Ser143 by AKT1 kinase. These two modifications are mutually exclusive, and structural analysis suggests that Ser143 phosphorylation interferes with Lys142 monomethylation. AKT1 kinase colocalizes and directly interacts with DNMT1 and phosphorylates Ser143. Phosphorylated DNMT1 peaks during DNA synthesis, before DNMT1 methylation. Depletion of AKT1 or overexpression of dominant-negative AKT1 increases methylated DNMT1, resulting in a decrease in DNMT1 abundance. In mammalian cells, phosphorylated DNMT1 is more stable than methylated DNMT1. These results reveal cross-talk on DNMT1, through modifications mediated by AKT1 and SET7, that affects cellular DNMT1 levels.
Organizational Affiliation:
New England Biolabs, Ipswich, Massachusetts, USA.