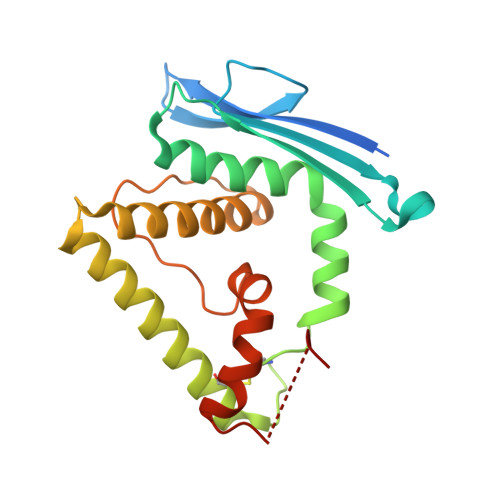
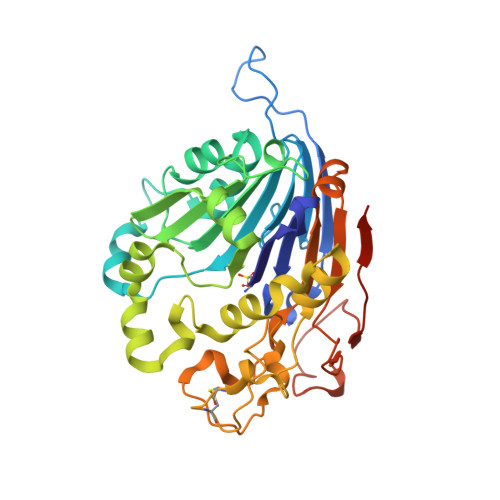
Initial insight into the function of the lysosomal 66.3 kDa protein from mouse by means of X-ray crystallography
Lakomek, K., Dickmanns, A., Kettwig, M., Urlaub, H., Ficner, R., Luebke, T.(2009) BMC Struct Biol 9: 56-56
- PubMed: 19706171
- DOI: https://doi.org/10.1186/1472-6807-9-56
- Primary Citation of Related Structures:
3FGR, 3FGT, 3FGW - PubMed Abstract:
The lysosomal 66.3 kDa protein from mouse is a soluble, mannose 6-phosphate containing protein of so far unknown function. It is synthesized as a glycosylated 75 kDa precursor that undergoes limited proteolysis leading to a 28 kDa N- and a 40 kDa C-terminal fragment.
Organizational Affiliation:
Department of Molecular Structural Biology, Institute of Microbiology and Genetics, GZMB, Georg-August University Goettingen, Justus-von-Liebig-Weg 11, D-37077 Goettingen, Germany. klakome@gwdg.de