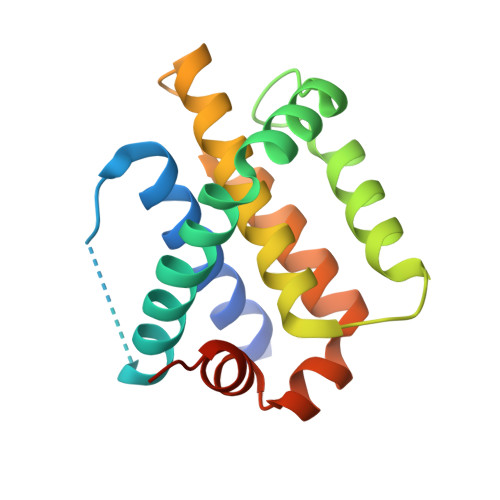
A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation.
Lee, E.F., Czabotar, P.E., van Delft, M.F., Michalak, E.M., Boyle, M.J., Willis, S.N., Puthalakath, H., Bouillet, P., Colman, P.M., Huang, D.C.S., Fairlie, W.D.(2008) J Cell Biol 180: 341-355
- PubMed: 18209102
- DOI: https://doi.org/10.1083/jcb.200708096
- Primary Citation of Related Structures:
3D7V - PubMed Abstract:
Like Bcl-2, Mcl-1 is an important survival factor for many cancers, its expression contributing to chemoresistance and disease relapse. However, unlike other prosurvival Bcl-2-like proteins, Mcl-1 stability is acutely regulated. For example, the Bcl-2 homology 3 (BH3)-only protein Noxa, which preferentially binds to Mcl-1, also targets it for proteasomal degradation. In this paper, we describe the discovery and characterization of a novel BH3-like ligand derived from Bim, Bim(S)2A, which is highly selective for Mcl-1. Unlike Noxa, Bim(S)2A is unable to trigger Mcl-1 degradation, yet, like Noxa, Bim(S)2A promotes cell killing only when Bcl-x(L) is absent or neutralized. Furthermore, killing by endogenous Bim is not associated with Mcl-1 degradation. Thus, functional inactivation of Mcl-1 does not always require its elimination. Rather, it can be efficiently antagonized by a BH3-like ligand tightly engaging its binding groove, which is confirmed here with a structural study. Our data have important implications for the discovery of compounds that might kill cells whose survival depends on Mcl-1.
Organizational Affiliation:
The Walter and Eliza Hall Institute of Medical Research, Parkville, Victoria 3050, Australia.