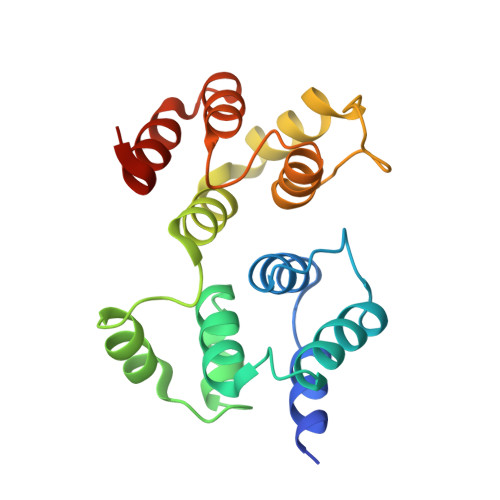
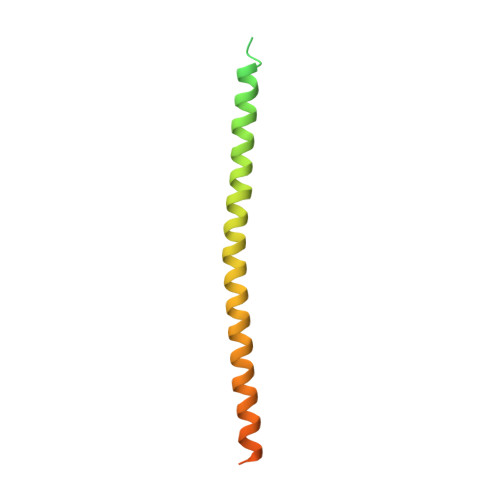
Crystal structure of a vFlip-IKKgamma complex: insights into viral activation of the IKK signalosome.
Bagneris, C., Ageichik, A.V., Cronin, N., Wallace, B., Collins, M., Boshoff, C., Waksman, G., Barrett, T.(2008) Mol Cell 30: 620-631
- PubMed: 18538660
- DOI: https://doi.org/10.1016/j.molcel.2008.04.029
- Primary Citation of Related Structures:
3CL3 - PubMed Abstract:
Key to the pathogenicity of several viruses is activation of the canonical nuclear factor-kappaB (NF-kappaB) transcriptional pathway. Subversion of this tightly regulated mechanism is achieved through the production of host mimetic viral proteins that deregulate the transcription process. One such protein is ks-vFLIP (produced by the Kaposi's sarcoma herpes virus [KSHV]), which associates with IKKgamma, an essential component of the IKK complex or signalosome. This interaction renders the canonical NF-kappaB pathway constitutively active and has been linked to Kaposi's sarcoma and other malignancies. In order to elucidate the molecular basis underpinning ks-vFLIP-induced activation of the IKK signalosome, we have determined the crystal structure of a complex involving a fragment of IKKgamma bound to ks-vFLIP at 3.2 A. In addition to identifying and subsequently probing the ks-vFLIP-IKKgamma interface, we have also investigated the effects of a mutation implicated in the genetic disorder anhydrotic ectodermal dysplasia with immunodeficiency (EDA-ID).
Organizational Affiliation:
Institute of Structural and Molecular Biology, School of Crystallography, Birkbeck College, Malet Street, London WC1E 7HX, UK.