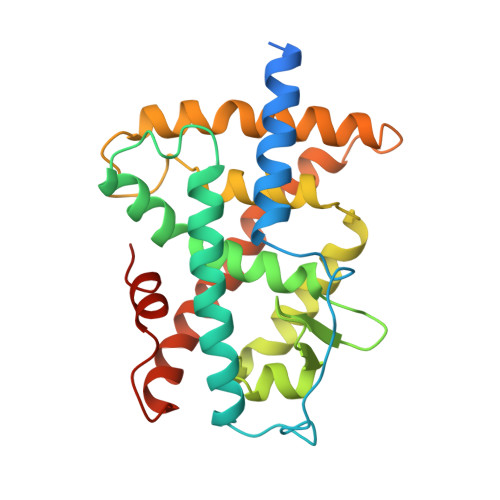
Crystal structure of the RAR-gamma ligand-binding domain bound to all-trans retinoic acid.
Renaud, J.P., Rochel, N., Ruff, M., Vivat, V., Chambon, P., Gronemeyer, H., Moras, D.(1995) Nature 378: 681-689
- PubMed: 7501014
- DOI: https://doi.org/10.1038/378681a0
- Primary Citation of Related Structures:
2LBD - PubMed Abstract:
The 2.0-A crystal structure of the ligand-binding domain (LBD) of the human retinoic acid receptor (RAR)-gamma bound to all-trans retinoic acid reveals the ligand-binding interactions and suggests an electrostatic guidance mechanism. The overall fold is similar to that of the human RXR-alpha apo-LBD, except for the carboxy-terminal part which folds back towards the LBD core, contributing to the hydrophobic ligand pocket and 'sealing' its entry site. We propose a 'mouse trap' mechanism whereby a ligand-induced conformational transition repositions the amphipathic alpha-helix of the AF-2 activating domain and forms a transcriptionally active receptor.
Organizational Affiliation:
Institut de Génétique et de Biologie Moléculaire et Cellulaire, CNRS/INSERM/ULP/Collège de France, Strasbourg, France.