Study of angiotensin-(1-7) vasoactive peptide and its beta-cyclodextrin inclusion complexes: complete sequence-specific NMR assignments and structural studies
Lula, I., Denadai, A.L., Resende, J.M., de Sousa, F.B., de Lima, G.F., Pilo-Veloso, D., Heine, T., Duarte, H.A., Santos, R.A., Sinisterra, R.D.(2007) Peptides 28: 2199-2210
- PubMed: 17904691
- DOI: https://doi.org/10.1016/j.peptides.2007.08.011
- Primary Citation of Related Structures:
2JP8 - PubMed Abstract:
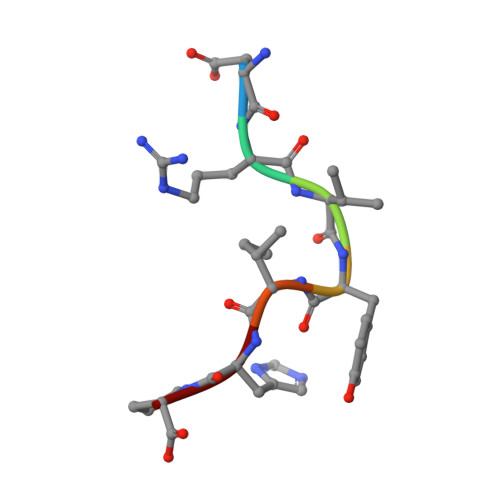
We report the complete sequence-specific hydrogen NMR assignments of vasoactive peptide angiotensin-(1-7) (Ang-(1-7)). Assignments of the majority of the resonances were accomplished by COSY, TOCSY, and ROESY peak coordinates at 400MHz and 600MHz. Long-side-chain amino acid spin system identification was facilitated by long-range coherence transfer experiments (TOCSY). Problems with overlapped resonance signals were solved by analysis of heteronuclear 2D experiments (HSQC and HMBC). Nuclear Overhauser effects (NOE) results were used to probe peptide conformation. We show that the inclusion of the angiotensin-(1-7) tyrosine residue is favored in inclusion complexes with beta-cyclodextrin. QM/MM simulations at the DFTB/UFF level confirm the experimental NMR findings and provide detailed structural information on these compounds in aqueous solution.
Organizational Affiliation:
Departamento de Química, Instituto de Ciências Exatas, Universidade Federal de Minas Gerais, Av. Antonio Carlos 6627, 31270-901 Belo Horizonte, Minas Gerais, Brazil.