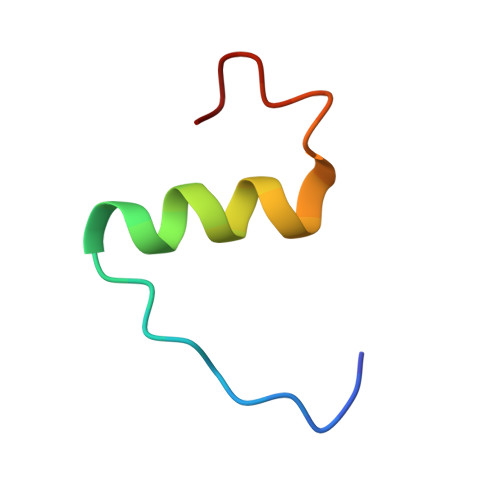
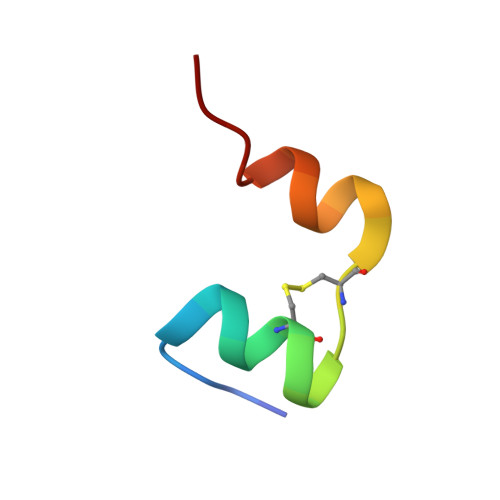
Solution Structure and Characterization of the LGR8 Receptor Binding Surface of Insulin-like Peptide 3
Rosengren, K.J., Zhang, S., Lin, F., Daly, N.L., Scott, D.J., Hughes, R.A., Bathgate, R.A.D., Craik, D.J., Wade, J.D.(2006) J Biol Chem 281: 28287-28295
- PubMed: 16867980
- DOI: https://doi.org/10.1074/jbc.M603829200
- Primary Citation of Related Structures:
2H8B - PubMed Abstract:
Insulin-like peptide 3 (INSL3), a member of the relaxin peptide family, is produced in testicular Leydig cells and ovarian thecal cells. Gene knock-out experiments have identified a key biological role in initiating testes descent during fetal development. Additionally, INSL3 has an important function in mediating male and female germ cell function. These actions are elicited via its recently identified receptor, LGR8, a member of the leucine-rich repeat-containing G-protein-coupled receptor family. To identify the structural features that are responsible for the interaction of INSL3 with its receptor, its solution structure was determined by NMR spectroscopy together with in vitro assays of a series of B-chain alanine-substituted analogs. Synthetic human INSL3 was found to adopt a characteristic relaxin/insulin-like fold in solution but is a highly dynamic molecule. The four termini of this two-chain peptide are disordered, and additional conformational exchange is evident in the molecular core. Alanine-substituted analogs were used to identify the key residues of INSL3 that are responsible for the interaction with the ectodomain of LGR8. These include Arg(B16) and Val(B19), with His(B12) and Arg(B20) playing a secondary role, as evident from the synergistic effect on the activity in double and triple mutants involving these residues. Together, these amino acids combine with the previously identified critical residue, Trp(B27), to form the receptor binding surface. The current results provide clear direction for the design of novel specific agonists and antagonists of this receptor.
Organizational Affiliation:
Department of Chemistry and Biomedical Sciences, University of Kalmar, SE-392 81 Kalmar, Sweden.