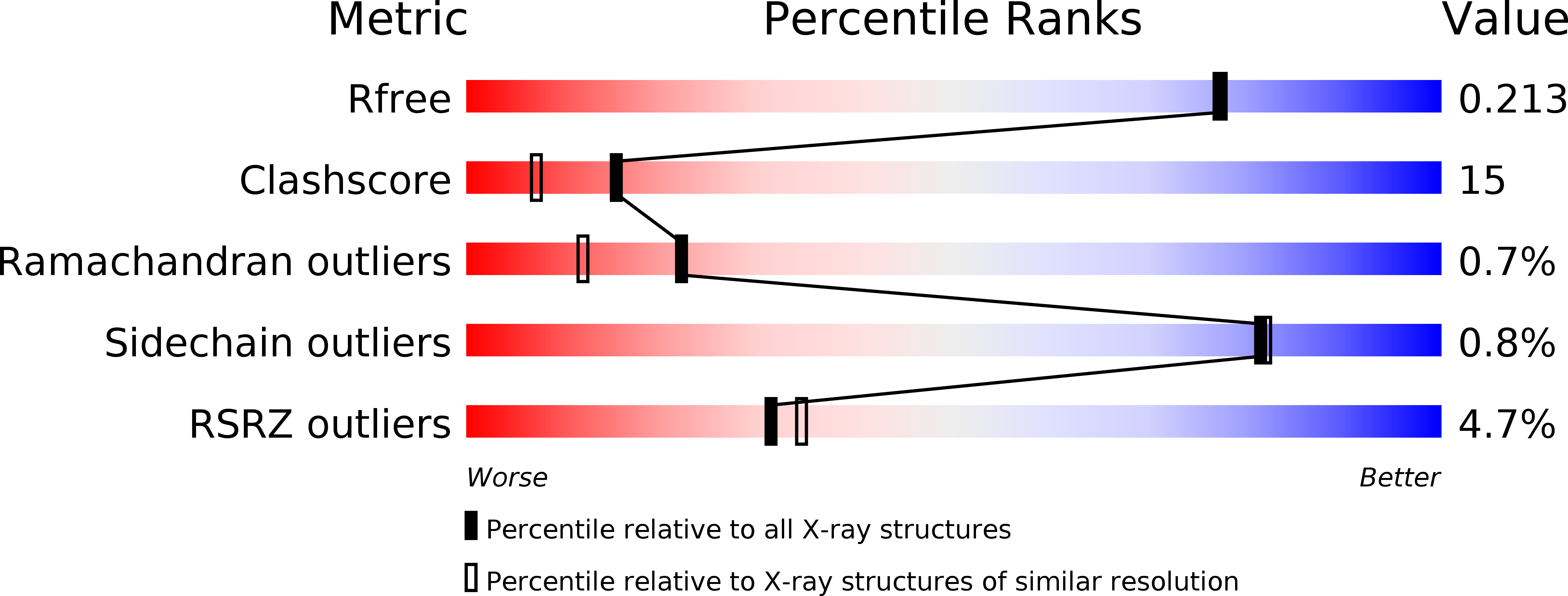
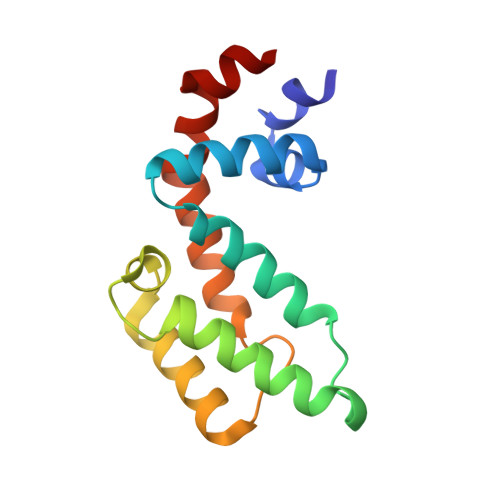
Structural basis of the Axin-adenomatous polyposis coli interaction.
Spink, K.E., Polakis, P., Weis, W.I.(2000) EMBO J 19: 2270-2279
- PubMed: 10811618
- DOI: https://doi.org/10.1093/emboj/19.10.2270
- Primary Citation of Related Structures:
1DK8, 1EMU - PubMed Abstract:
Axin and the adenomatous polyposis coli (APC) tumor suppressor protein are components of the Wnt/Wingless growth factor signaling pathway. In the absence of Wnt signal, Axin and APC regulate cytoplasmic levels of the proto-oncogene beta-catenin through the formation of a large complex containing these three proteins, glycogen synthase kinase 3beta (GSK3beta) and several other proteins. Both Axin and APC are known to be critical for beta-catenin regulation, and truncations in APC that eliminate the Axin-binding site result in human cancers. A protease-resistant domain of Axin that contains the APC-binding site is a member of the regulators of G-protein signaling (RGS) superfamily. The crystal structures of this domain alone and in complex with an Axin-binding sequence from APC reveal that the Axin-APC interaction occurs at a conserved groove on a face of the protein that is distinct from the G-protein interface of classical RGS proteins. The molecular interactions observed in the Axin-APC complex provide a rationale for the evolutionary conservation seen in both proteins.
Organizational Affiliation:
Department of Structural Biology, Stanford University School of Medicine, 299 Campus Drive West, Stanford, CA 94305, USA.