Structural insights into a novel nonheme iron-dependent oxygenase in selenoneine biosynthesis.
Liu, M., Yang, Y., Huang, J.W., Dai, L., Zheng, Y., Cheng, S., He, H., Chen, C.C., Guo, R.T.(2023) Int J Biol Macromol 256: 128428-128428
- PubMed: 38013086
- DOI: https://doi.org/10.1016/j.ijbiomac.2023.128428
- Primary Citation of Related Structures:
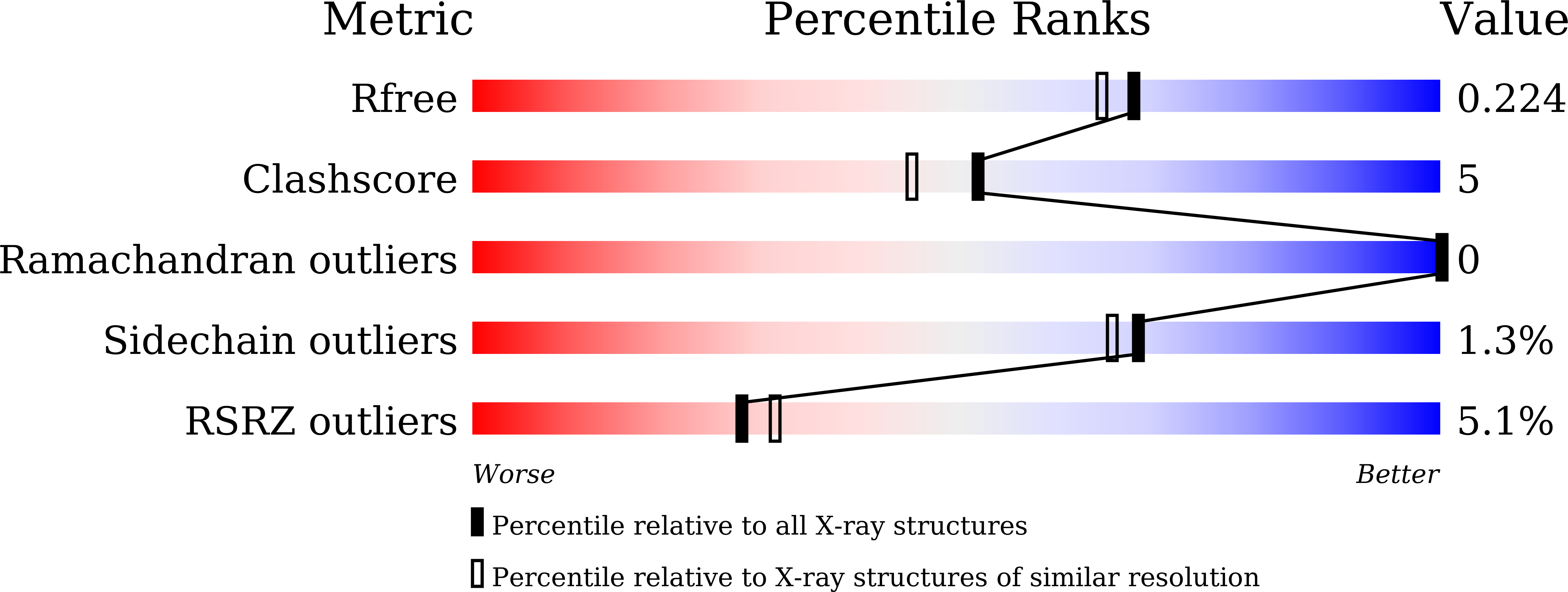
8K5I, 8K5J, 8K5K - PubMed Abstract:
Selenoneine (SEN) is a natural histidine derivative with radical-scavenging activity and shows higher antioxidant potential than its sulfur-containing isolog ergothioneine (EGT). Recently, the SEN biosynthetic pathway in Variovorax paradoxus was reported. Resembling EGT biosynthesis, the committed step of SEN synthesis is catalyzed by a nonheme Fe-dependent oxygenase termed SenA. This enzyme catalyzes oxidative carbon‑selenium (C-Se) bond formation to conjugate N-α-trimethyl histidine (TMH) and selenosugar to yield selenoxide; the process parallels the EGT biosynthetic route, in which sulfoxide synthases known as EgtB members catalyze the conjugation of TMH and cysteine or γ-glutamylcysteine to afford sulfoxides. Here, we report the crystal structures of SenA and its complex with TMH and thioglucose (SGlc), an analog of selenoglucose (SeGlc) at high resolution. The overall structure of SenA adopts the archetypical fold of EgtB, which comprises a DinB-like domain and an FGE-like domain. While the TMH-binding site is highly conserved to that of EgtB, a various substrate-enzyme interaction network in the selenosugar-binding site of SenA features a number of water-mediated hydrogen bonds. The obtained structural information is beneficial for understanding the mechanism of SenA-mediated C-Se bond formation.
Organizational Affiliation:
State Key Laboratory of Biocatalysis and Enzyme Engineering, Hubei Hongshan Laboratory, Hubei Collaborative Innovation Center for Green Transformation of Bio-Resources, Hubei Key Laboratory of Industrial Biotechnology, School of Life Sciences, Hubei University, Wuhan, 430062, China.