Cryo-EM structure ofABCC4
Chen, Y., Wang, L., Hou, W.T., Zhou, C.Z., Chen, Y., Li, Q.(2023) Nat Cardiovasc Res
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| ATP-binding cassette sub-family C member 4 | 1,325 | Homo sapiens | Mutation(s): 0 Gene Names: ABCC4, MOATB, MRP4 EC: 7.6.2 (PDB Primary Data), 7.6.2.2 (PDB Primary Data), 7.6.2.3 (PDB Primary Data) Membrane Entity: Yes | 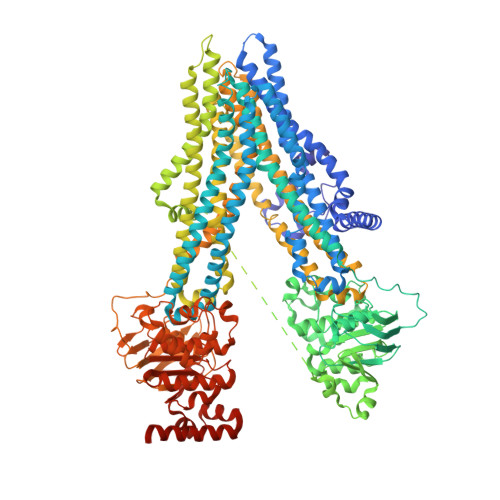 | |
UniProt & NIH Common Fund Data Resources | |||||
Find proteins for O15439 (Homo sapiens) Explore O15439 Go to UniProtKB: O15439 | |||||
PHAROS: O15439 GTEx: ENSG00000125257 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | O15439 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 1 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| AIN (Subject of Investigation/LOI) Query on AIN | B [auth A] | 2-(ACETYLOXY)BENZOIC ACID C9 H8 O4 BSYNRYMUTXBXSQ-UHFFFAOYSA-N |  | ||
| Task | Software Package | Version |
|---|---|---|
| MODEL REFINEMENT | PHENIX | 1.18.2_3874: |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Ministry of Science and Technology (MoST, China) | China | 2019YFA0508500 |
| Chinese Academy of Sciences | China | XDB37020202 |