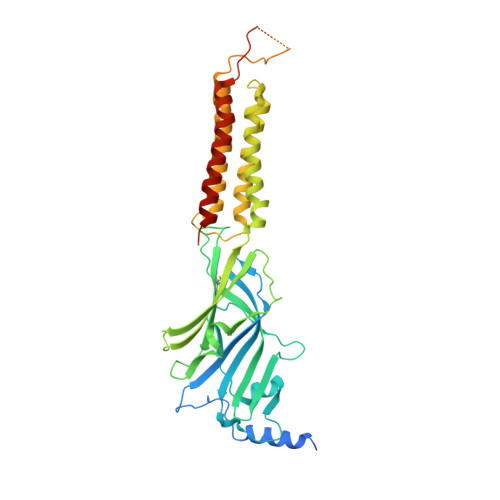
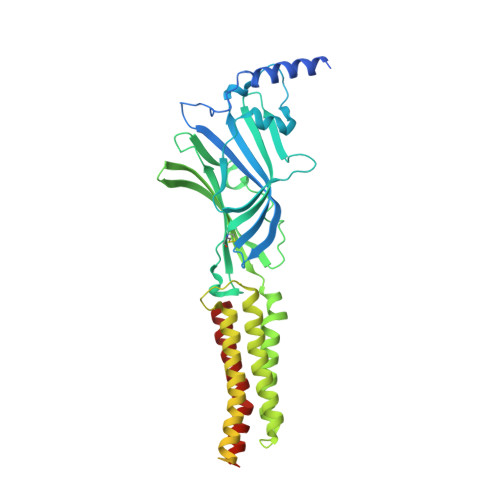
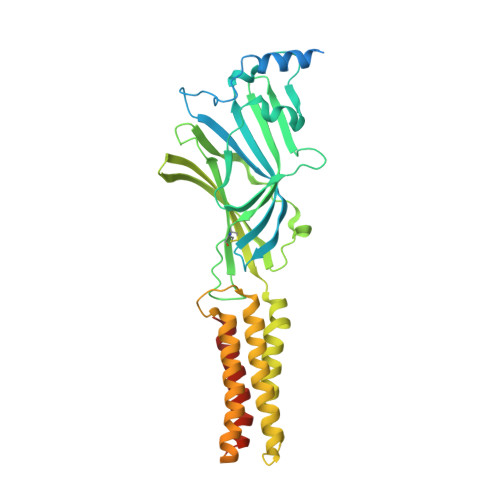
Cryo-EM structures reveal native GABA A receptor assemblies and pharmacology.
Sun, C., Zhu, H., Clark, S., Gouaux, E.(2023) Nature 622: 195-201
- PubMed: 37730991
- DOI: https://doi.org/10.1038/s41586-023-06556-w
- Primary Citation of Related Structures:
8FOI, 8G4N, 8G4O, 8G4X, 8G5F, 8G5G, 8G5H - PubMed Abstract:
Type A γ-aminobutyric acid receptors (GABA A Rs) are the principal inhibitory receptors in the brain and the target of a wide range of clinical agents, including anaesthetics, sedatives, hypnotics and antidepressants 1-3 . However, our understanding of GABA A R pharmacology has been hindered by the vast number of pentameric assemblies that can be derived from 19 different subunits 4 and the lack of structural knowledge of clinically relevant receptors. Here, we isolate native murine GABA A R assemblies containing the widely expressed α1 subunit and elucidate their structures in complex with drugs used to treat insomnia (zolpidem (ZOL) and flurazepam) and postpartum depression (the neurosteroid allopregnanolone (APG)). Using cryo-electron microscopy (cryo-EM) analysis and single-molecule photobleaching experiments, we uncover three major structural populations in the brain: the canonical α1β2γ2 receptor containing two α1 subunits, and two assemblies containing one α1 and either an α2 or α3 subunit, in which the single α1-containing receptors feature a more compact arrangement between the transmembrane and extracellular domains. Interestingly, APG is bound at the transmembrane α/β subunit interface, even when not added to the sample, revealing an important role for endogenous neurosteroids in modulating native GABA A Rs. Together with structurally engaged lipids, neurosteroids produce global conformational changes throughout the receptor that modify the ion channel pore and the binding sites for GABA and insomnia medications. Our data reveal the major α1-containing GABA A R assemblies, bound with endogenous neurosteroid, thus defining a structural landscape from which subtype-specific drugs can be developed.
Organizational Affiliation:
Vollum Institute, Oregon Health and Science University, Portland, OR, USA.