Structural insight to elucidate the binding specificity of the anti-cortisol Fab fragment with glucocorticoids.
Eronen, V., Tullila, A., Iljin, K., Rouvinen, J., Nevanen, T.K., Hakulinen, N.(2023) J Struct Biol 215: 107966-107966
- PubMed: 37100101
- DOI: https://doi.org/10.1016/j.jsb.2023.107966
- Primary Citation of Related Structures:
8CBX, 8CBY, 8CBZ, 8CC0, 8CC1 - PubMed Abstract:
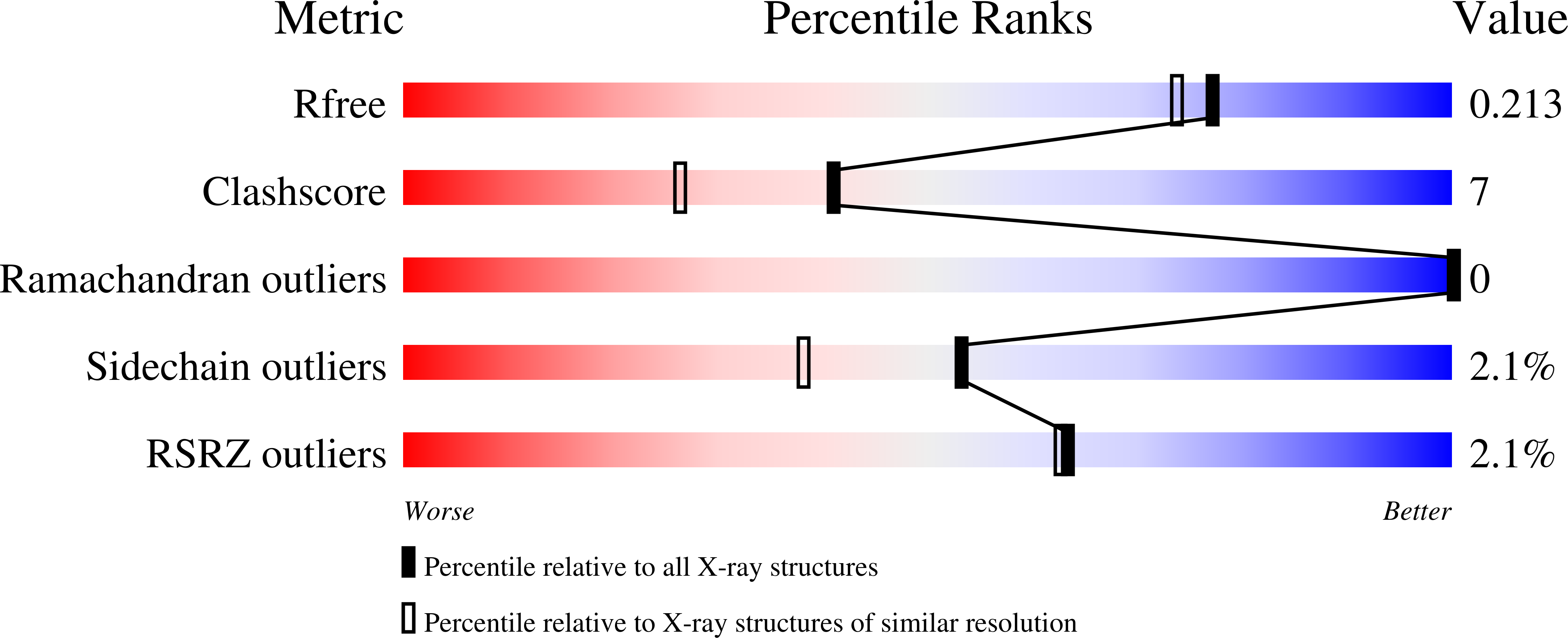
Cortisol is a steroid hormone that is produced by the adrenal gland. It is a primary stress hormone that increases glucose levels in the blood stream. High concentrations of cortisol in the body can be used as a biomarker for acute and chronic stress and related mental and physiological disorders. Therefore, the accurate quantification of cortisol levels in body fluids is essential for clinical diagnosis. In this article, we describe the isolation of recombinant anti-cortisol antibodies with high affinity for cortisol and discover their cross-reactivity with other glucocorticoids. To describe the cortisol binding site and elucidate the structural basis for the binding specificity, the high-resolution crystal structures of the anti-cortisol (17) Fab fragment in the absence of glucocorticoid (2.00 Å) and the presence of cortisol (2.26 Å), corticosterone (1.86 Å), cortisone (1.85 Å) and prednisolone (2.00 Å) were determined. To our knowledge, this is the first determined crystal structure of a cortisol-specific antibody. The recognition of cortisol is driven by hydrophobic interactions and hydrogen bonding at the protein-ligand interface coupled with a conformational transition. Comparison of ligand-free and ligand-bound structures showed that the side chains of residues Tyr58-H and Arg56-H can undergo local conformational changes at the binding site, most likely prior to the binding event via a conformational selection mechanism. Compared to other anti-steroid antibody-antigen complexes, (17) Fab possesses a structurally unique steroid binding site, as the H3 loop from the CDR area has only a minor contribution, but framework residues have a prominent contribution to hapten binding.
Organizational Affiliation:
Department of Chemistry, University of Eastern Finland, PO BOX 111, 80100 Joensuu, Finland.