Aster-dependent nonvesicular transport facilitates dietary cholesterol uptake.
Ferrari, A., Whang, E., Xiao, X., Kennelly, J.P., Romartinez-Alonso, B., Mack, J.J., Weston, T., Chen, K., Kim, Y., Tol, M.J., Bideyan, L., Nguyen, A., Gao, Y., Cui, L., Bedard, A.H., Sandhu, J., Lee, S.D., Fairall, L., Williams, K.J., Song, W., Munguia, P., Russell, R.A., Martin, M.G., Jung, M.E., Jiang, H., Schwabe, J.W.R., Young, S.G., Tontonoz, P.(2023) Science 382: eadf0966-eadf0966
- PubMed: 37943936
- DOI: https://doi.org/10.1126/science.adf0966
- Primary Citation of Related Structures:
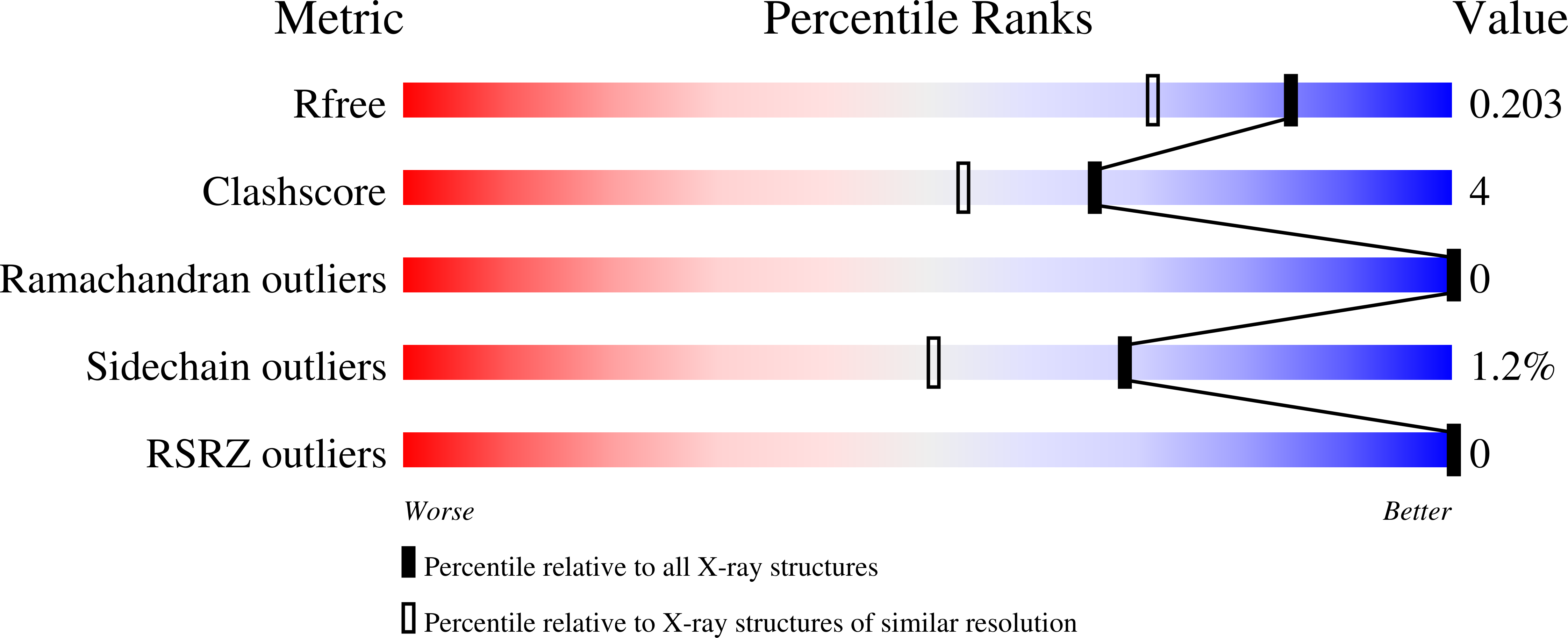
8AXW - PubMed Abstract:
Intestinal absorption is an important contributor to systemic cholesterol homeostasis. Niemann-Pick C1 Like 1 (NPC1L1) assists in the initial step of dietary cholesterol uptake, but how cholesterol moves downstream of NPC1L1 is unknown. We show that Aster-B and Aster-C are critical for nonvesicular cholesterol movement in enterocytes. Loss of NPC1L1 diminishes accessible plasma membrane (PM) cholesterol and abolishes Aster recruitment to the intestinal brush border. Enterocytes lacking Asters accumulate PM cholesterol and show endoplasmic reticulum cholesterol depletion. Aster-deficient mice have impaired cholesterol absorption and are protected against diet-induced hypercholesterolemia. Finally, the Aster pathway can be targeted with a small-molecule inhibitor to manipulate cholesterol uptake. These findings identify the Aster pathway as a physiologically important and pharmacologically tractable node in dietary lipid absorption.
Organizational Affiliation:
Department of Pathology and Laboratory Medicine, University of California, Los Angeles, Los Angeles, CA 90095, USA.