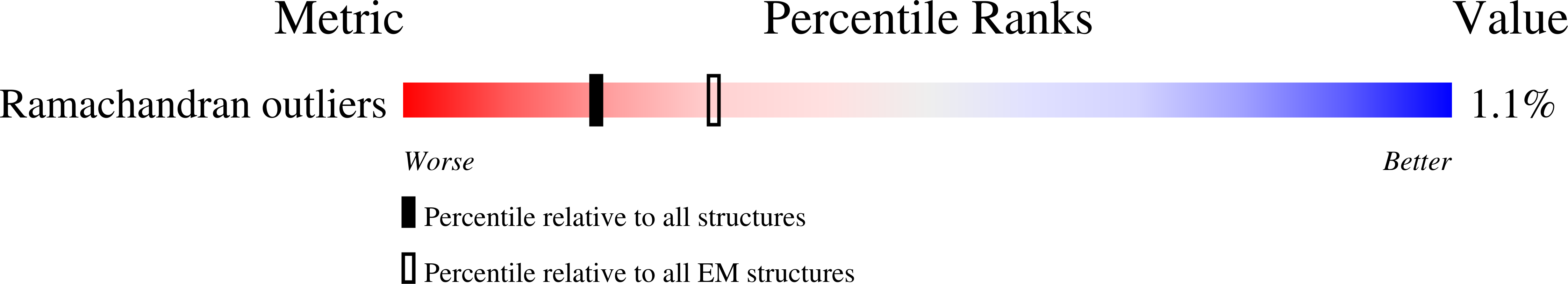Elucidating interprotein energy transfer dynamics within the antenna network from purple bacteria.
Wang, D., Fiebig, O.C., Harris, D., Toporik, H., Ji, Y., Chuang, C., Nairat, M., Tong, A.L., Ogren, J.I., Hart, S.M., Cao, J., Sturgis, J.N., Mazor, Y., Schlau-Cohen, G.S.(2023) Proc Natl Acad Sci U S A 120: e2220477120-e2220477120
- PubMed: 37399405
- DOI: https://doi.org/10.1073/pnas.2220477120
- Primary Citation of Related Structures:
7TUW, 7TV3, 8FB9, 8FBB - PubMed Abstract:
In photosynthesis, absorbed light energy transfers through a network of antenna proteins with near-unity quantum efficiency to reach the reaction center, which initiates the downstream biochemical reactions. While the energy transfer dynamics within individual antenna proteins have been extensively studied over the past decades, the dynamics between the proteins are poorly understood due to the heterogeneous organization of the network. Previously reported timescales averaged over such heterogeneity, obscuring individual interprotein energy transfer steps. Here, we isolated and interrogated interprotein energy transfer by embedding two variants of the primary antenna protein from purple bacteria, light-harvesting complex 2 (LH2), together into a near-native membrane disc, known as a nanodisc. We integrated ultrafast transient absorption spectroscopy, quantum dynamics simulations, and cryogenic electron microscopy to determine interprotein energy transfer timescales. By varying the diameter of the nanodiscs, we replicated a range of distances between the proteins. The closest distance possible between neighboring LH2, which is the most common in native membranes, is 25 Å and resulted in a timescale of 5.7 ps. Larger distances of 28 to 31 Å resulted in timescales of 10 to 14 ps. Corresponding simulations showed that the fast energy transfer steps between closely spaced LH2 increase transport distances by ∼15%. Overall, our results introduce a framework for well-controlled studies of interprotein energy transfer dynamics and suggest that protein pairs serve as the primary pathway for the efficient transport of solar energy.
Organizational Affiliation:
Department of Chemistry, Massachusetts Institute of Technology, Cambridge, MA 02139.