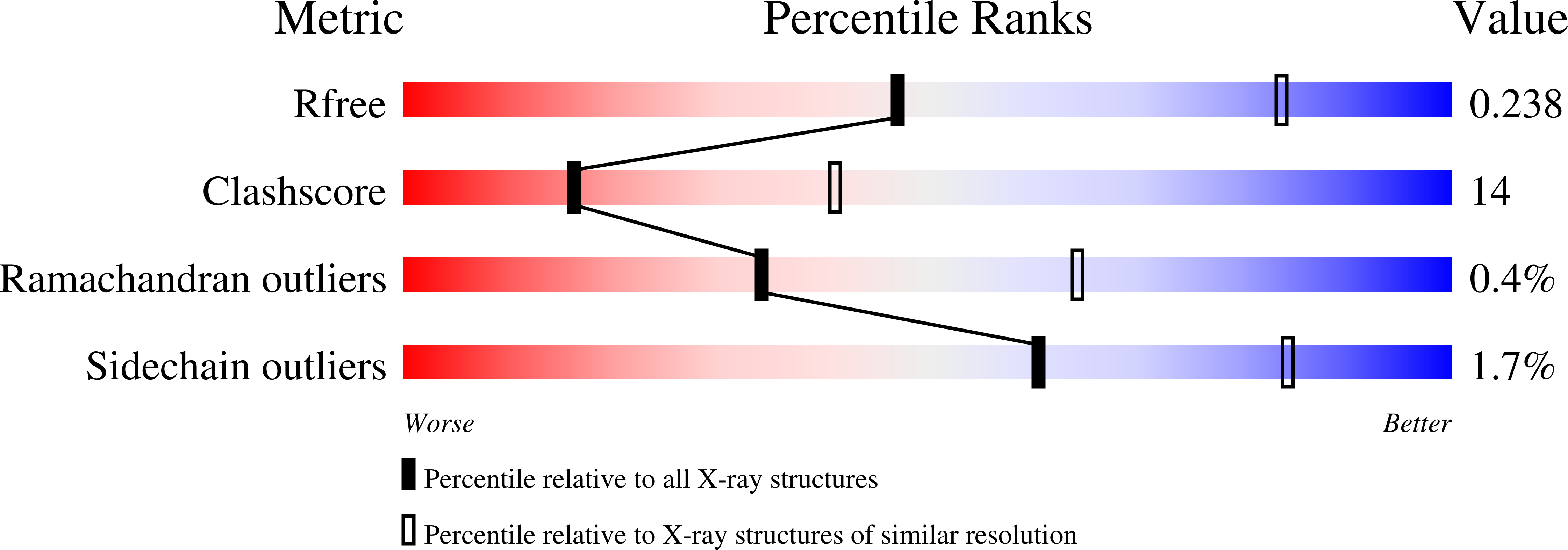
Clearing the Path to Rapid High-Quality Protein Purification
Chen, X., Wendorff, T., Jao, C., Kung, J.E., Liau, N.P.D., Wu, P., Yu, C., Oh, A., Boenig, G., Wallweber, H., Everett, C., Miu, A., Brillantes, B., Mortara, K., Murray, J., Koth, C.M., Sudhamsu, J.To be published.