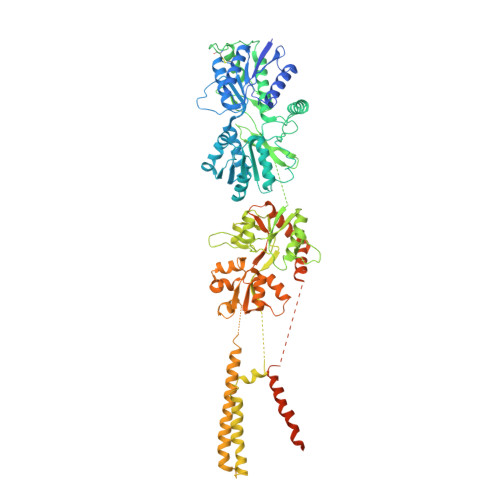
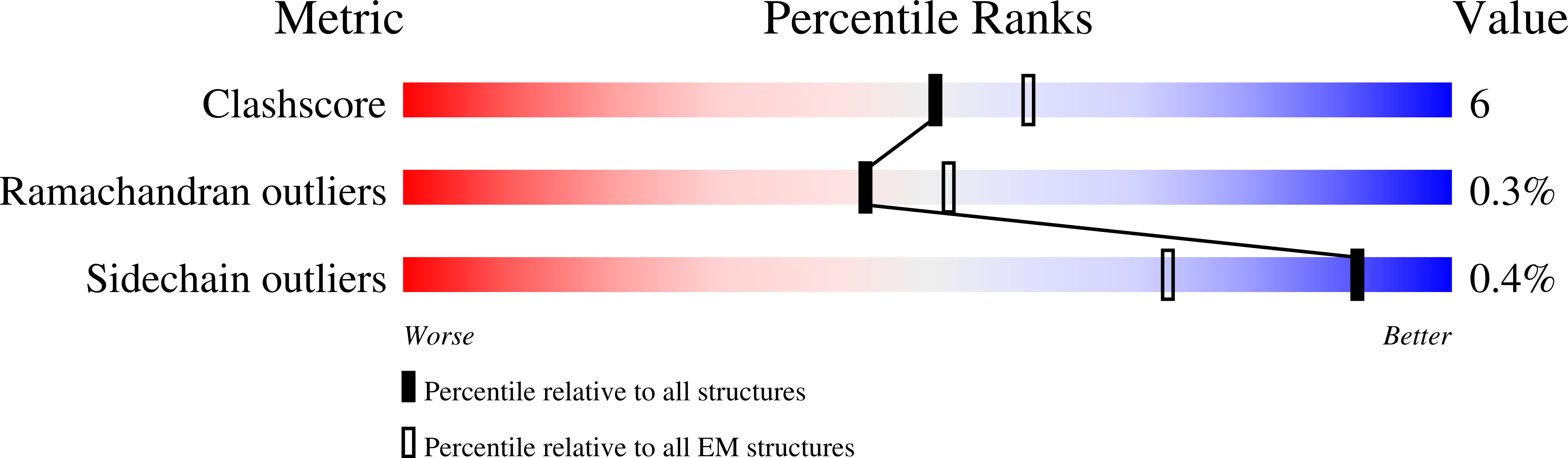Structural and compositional diversity in the kainate receptor family.
Selvakumar, P., Lee, J., Khanra, N., He, C., Munguba, H., Kiese, L., Broichhagen, J., Reiner, A., Levitz, J., Meyerson, J.R.(2021) Cell Rep 37: 109891-109891
- PubMed: 34706237
- DOI: https://doi.org/10.1016/j.celrep.2021.109891
- Primary Citation of Related Structures:
7LVT - PubMed Abstract:
The kainate receptors (KARs) are members of the ionotropic glutamate receptor family and assemble into tetramers from a pool of five subunit types (GluK1-5). Each subunit confers distinct functional properties to a receptor, but the compositional and stoichiometric diversity of KAR tetramers is not well understood. To address this, we first solve the structure of the GluK1 homomer, which enables a systematic assessment of structural compatibility among KAR subunits. Next, we analyze single-cell RNA sequencing data, which reveal extreme diversity in the combinations of two or more KAR subunits co-expressed within the same cell. We then investigate the composition of individual receptor complexes using single-molecule fluorescence techniques and find that di-heteromers assembled from GluK1, GluK2, or GluK3 can form with all possible stoichiometries, while GluK1/K5, GluK2/K5, and GluK3/K5 can form 3:1 or 2:2 complexes. Finally, using three-color single-molecule imaging, we discover that KARs can form tri- and tetra-heteromers.
Organizational Affiliation:
Department of Physiology and Biophysics, Weill Cornell Medical College, New York, NY 10065, USA.