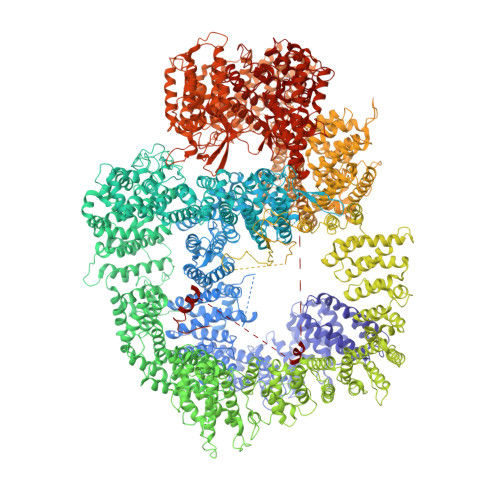
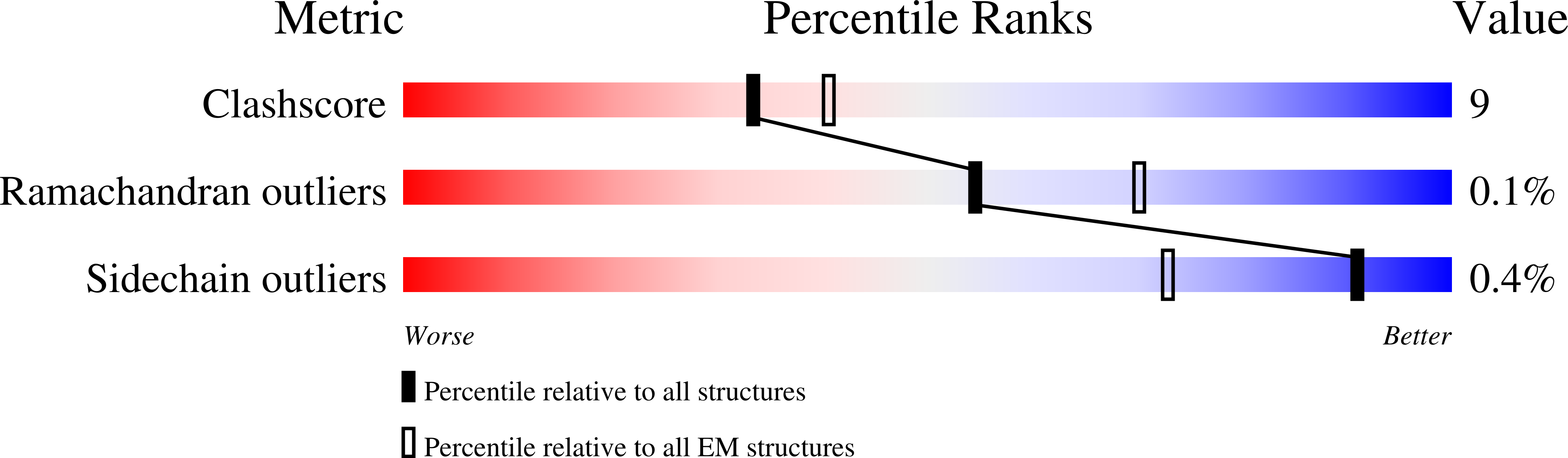Dimers of DNA-PK create a stage for DNA double-strand break repair.
Chaplin, A.K., Hardwick, S.W., Liang, S., Kefala Stavridi, A., Hnizda, A., Cooper, L.R., De Oliveira, T.M., Chirgadze, D.Y., Blundell, T.L.(2021) Nat Struct Mol Biol 28: 13-19
- PubMed: 33077952
- DOI: https://doi.org/10.1038/s41594-020-00517-x
- Primary Citation of Related Structures:
6ZFP, 6ZH2, 6ZH4, 6ZH6, 6ZH8, 6ZHA, 6ZHE - PubMed Abstract:
DNA double-strand breaks are the most dangerous type of DNA damage and, if not repaired correctly, can lead to cancer. In humans, Ku70/80 recognizes DNA broken ends and recruits the DNA-dependent protein kinase catalytic subunit (DNA-PKcs) to form DNA-dependent protein kinase holoenzyme (DNA-PK) in the process of non-homologous end joining (NHEJ). We present a 2.8-Å-resolution cryo-EM structure of DNA-PKcs, allowing precise amino acid sequence registration in regions uninterpreted in previous 4.3-Å X-ray maps. We also report a cryo-EM structure of DNA-PK at 3.5-Å resolution and reveal a dimer mediated by the Ku80 C terminus. Central to dimer formation is a domain swap of the conserved C-terminal helix of Ku80. Our results suggest a new mechanism for NHEJ utilizing a DNA-PK dimer to bring broken DNA ends together. Furthermore, drug inhibition of NHEJ in combination with chemo- and radiotherapy has proved successful, making these models central to structure-based drug targeting efforts.
Organizational Affiliation:
Department of Biochemistry, University of Cambridge, Cambridge, UK.