Allosteric inhibitors of Mycobacterium tuberculosis tryptophan synthase.
Michalska, K., Chang, C., Maltseva, N.I., Jedrzejczak, R., Robertson, G.T., Gusovsky, F., McCarren, P., Schreiber, S.L., Nag, P.P., Joachimiak, A.(2020) Protein Sci 29: 779-788
- PubMed: 31930594
- DOI: https://doi.org/10.1002/pro.3825
- Primary Citation of Related Structures:
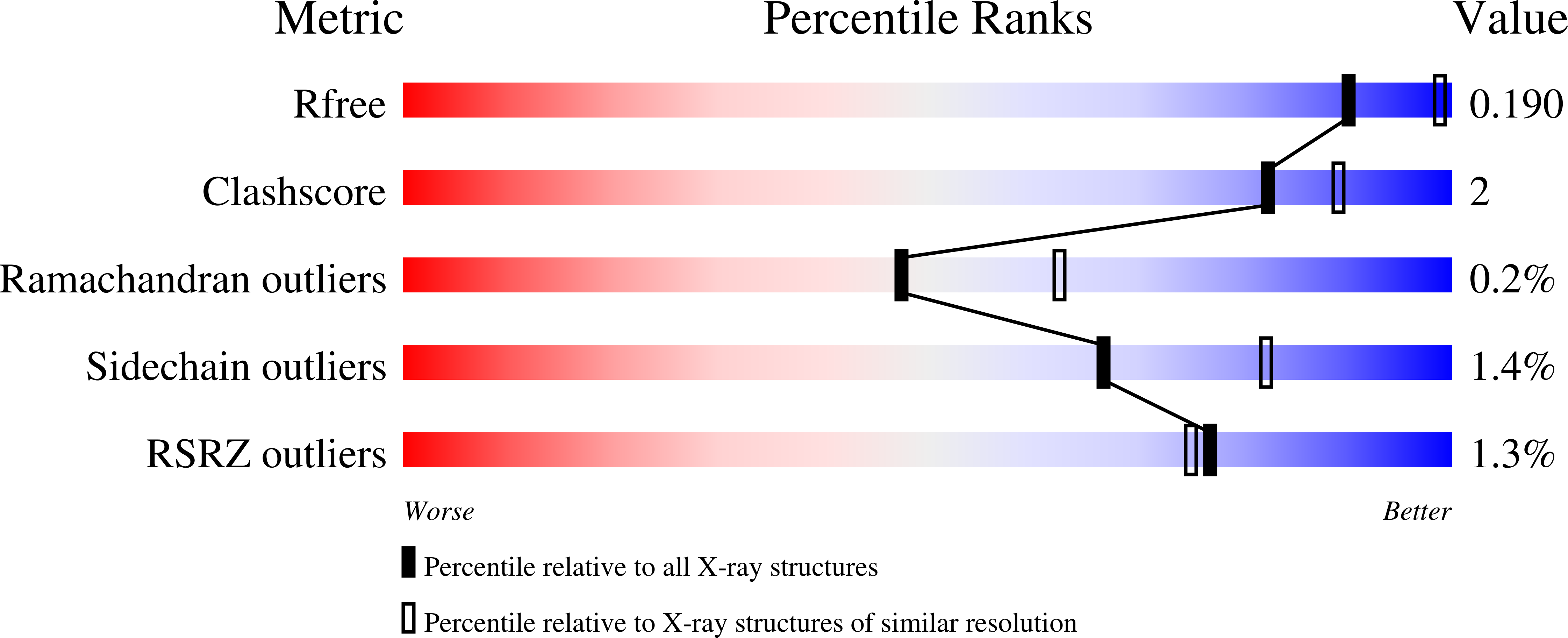
6U6C, 6USA - PubMed Abstract:
Global dispersion of multidrug resistant bacteria is very common and evolution of antibiotic-resistance is occurring at an alarming rate, presenting a formidable challenge for humanity. The development of new therapeuthics with novel molecular targets is urgently needed. Current drugs primarily affect protein, nucleic acid, and cell wall synthesis. Metabolic pathways, including those involved in amino acid biosynthesis, have recently sparked interest in the drug discovery community as potential reservoirs of such novel targets. Tryptophan biosynthesis, utilized by bacteria but absent in humans, represents one of the currently studied processes with a therapeutic focus. It has been shown that tryptophan synthase (TrpAB) is required for survival of Mycobacterium tuberculosis in macrophages and for evading host defense, and therefore is a promising drug target. Here we present crystal structures of TrpAB with two allosteric inhibitors of M. tuberculosis tryptophan synthase that belong to sulfolane and indole-5-sulfonamide chemical scaffolds. We compare our results with previously reported structural and biochemical studies of another, azetidine-containing M. tuberculosis tryptophan synthase inhibitor. This work shows how structurally distinct ligands can occupy the same allosteric site and make specific interactions. It also highlights the potential benefit of targeting more variable allosteric sites of important metabolic enzymes.
Organizational Affiliation:
Center for Structural Genomics of Infectious Diseases, Consortium for Advanced Science and Engineering, University of Chicago, Chicago, Illinois.