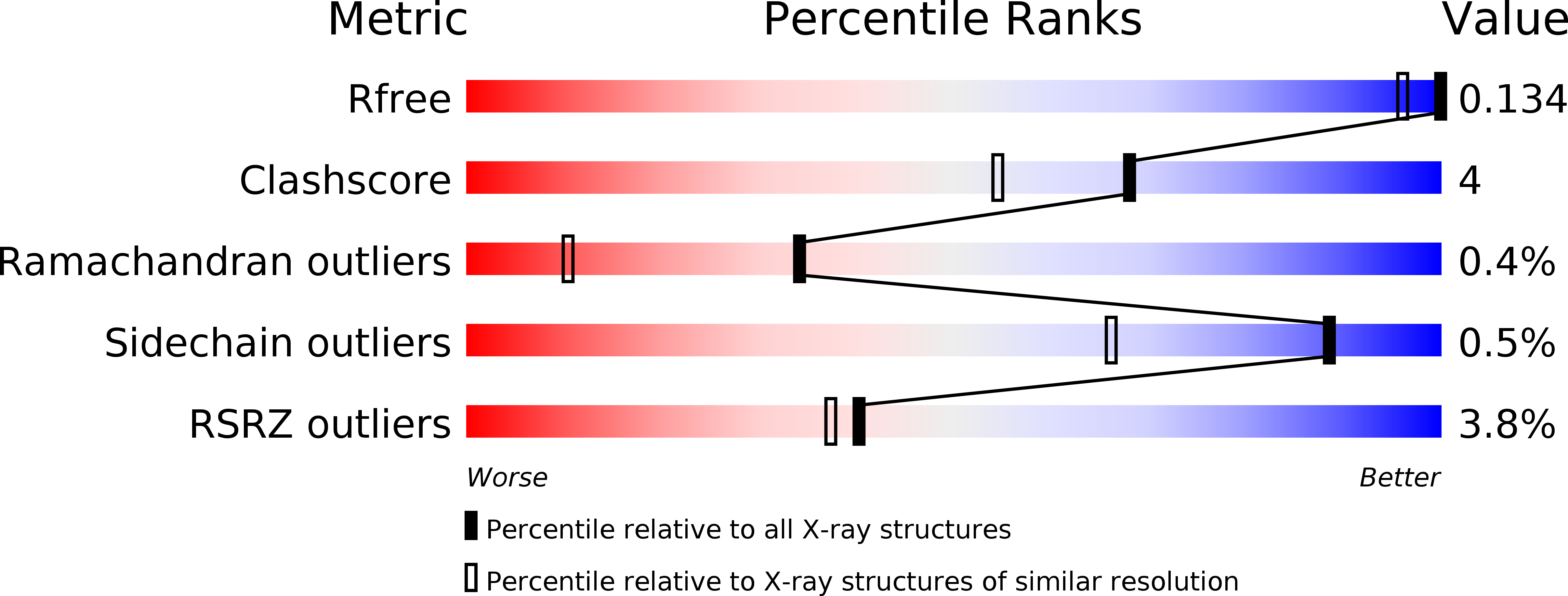
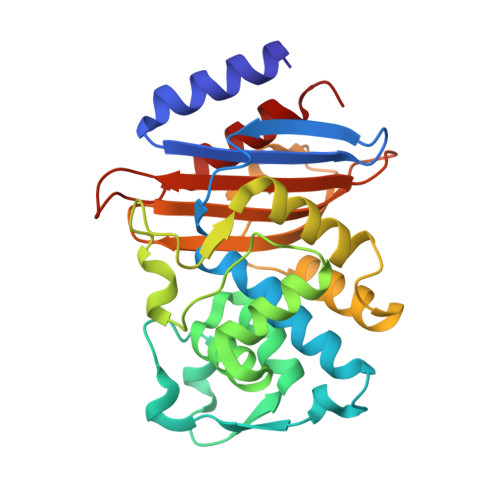
Discovery of Taniborbactam (VNRX-5133): A Broad-Spectrum Serine- and Metallo-beta-lactamase Inhibitor for Carbapenem-Resistant Bacterial Infections.
Liu, B., Trout, R.E.L., Chu, G.H., McGarry, D., Jackson, R.W., Hamrick, J.C., Daigle, D.M., Cusick, S.M., Pozzi, C., De Luca, F., Benvenuti, M., Mangani, S., Docquier, J.D., Weiss, W.J., Pevear, D.C., Xerri, L., Burns, C.J.(2020) J Med Chem 63: 2789-2801
- PubMed: 31765155
- DOI: https://doi.org/10.1021/acs.jmedchem.9b01518
- Primary Citation of Related Structures:
6SP6, 6SP7 - PubMed Abstract:
A major resistance mechanism in Gram-negative bacteria is the production of β-lactamase enzymes. Originally recognized for their ability to hydrolyze penicillins, emergent β-lactamases can now confer resistance to other β-lactam drugs, including both cephalosporins and carbapenems. The emergence and global spread of β-lactamase-producing multi-drug-resistant "superbugs" has caused increased alarm within the medical community due to the high mortality rate associated with these difficult-to-treat bacterial infections. To address this unmet medical need, we initiated an iterative program combining medicinal chemistry, structural biology, biochemical testing, and microbiological profiling to identify broad-spectrum inhibitors of both serine- and metallo-β-lactamase enzymes. Lead optimization, beginning with narrower-spectrum, weakly active compounds, provided 20 (VNRX-5133, taniborbactam), a boronic-acid-containing pan-spectrum β-lactamase inhibitor. In vitro and in vivo studies demonstrated that 20 restored the activity of β-lactam antibiotics against carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Enterobacteriaceae. Taniborbactam is the first pan-spectrum β-lactamase inhibitor to enter clinical development.
Organizational Affiliation:
Venatorx Pharmaceuticals, Inc., 30 Spring Mill Drive, Malvern, Pennsylvania 19355, United States.