Mycobacterial and Human Nitrobindins: Structure and Function.
De Simone, G., di Masi, A., Vita, G.M., Polticelli, F., Pesce, A., Nardini, M., Bolognesi, M., Ciaccio, C., Coletta, M., Turilli, E.S., Fasano, M., Tognaccini, L., Smulevich, G., Abbruzzetti, S., Viappiani, C., Bruno, S., Ascenzi, P.(2020) Antioxid Redox Signal 33: 229-246
- PubMed: 32295384
- DOI: https://doi.org/10.1089/ars.2019.7874
- Primary Citation of Related Structures:
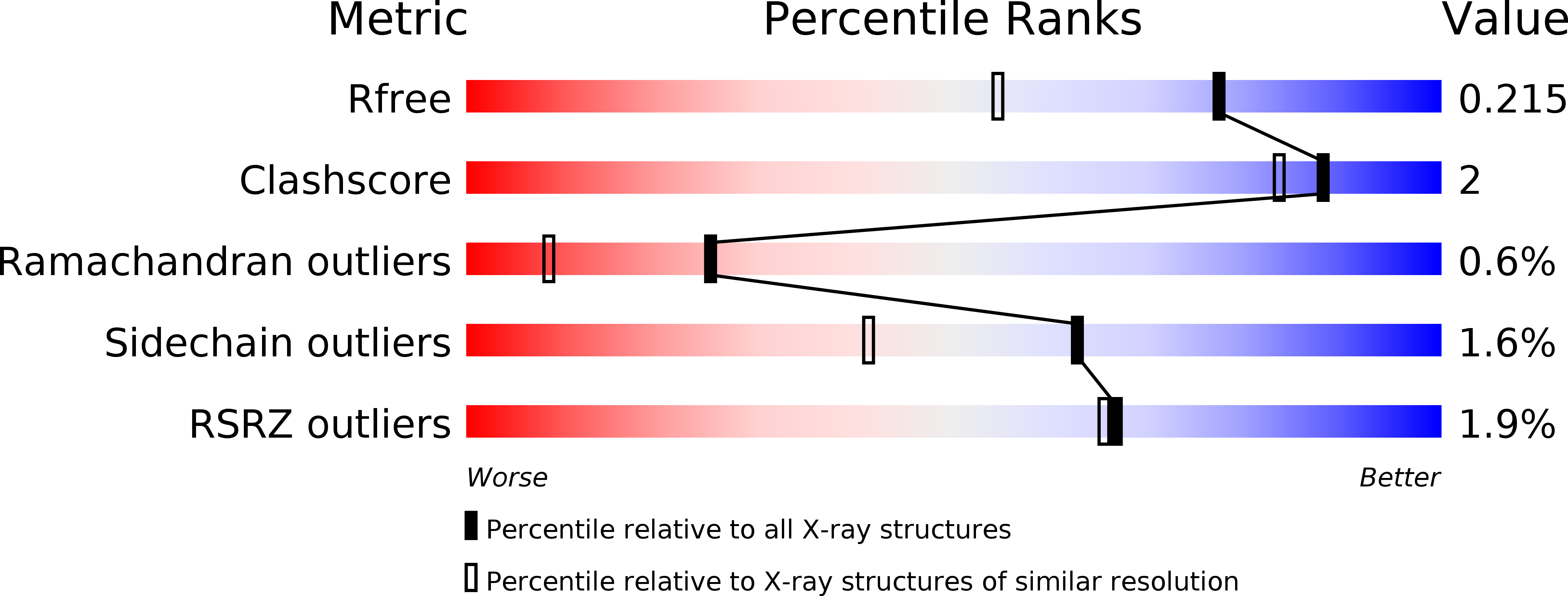
6R3W, 6R3Y - PubMed Abstract:
Aims: Nitrobindins (Nbs) are evolutionary conserved all-β-barrel heme-proteins displaying a highly solvent-exposed heme-Fe(III) atom. The physiological role(s) of Nbs is almost unknown. Here, the structural and functional properties of ferric Mycobacterium tuberculosis Nb ( Mt -Nb(III)) and ferric Homo sapiens Nb ( Hs -Nb(III)) have been investigated and compared with those of ferric Arabidopsis thaliana Nb ( At -Nb(III), Rhodnius prolixus nitrophorins ( Rp -NP(III)s), and mammalian myoglobins. Results: Data here reported demonstrate that Mt -Nb(III), At -Nb(III), and Hs -Nb(III) share with Rp -NP(III)s the capability to bind selectively nitric oxide, but display a very low reactivity, if any, toward histamine. Data obtained overexpressing Hs -Nb in human embryonic kidney 293 cells indicate that Hs -Nb localizes mainly in the cytoplasm and partially in the nucleus, thanks to a nuclear localization sequence encompassing residues Glu124-Leu154. Human Hs -Nb corresponds to the C -terminal domain of the human nuclear protein THAP4 suggesting that Nb may act as a sensor possibly modulating the THAP4 transcriptional activity residing in the N -terminal region. Finally, we provide strong evidence that both Mt -Nb(III) and Hs -Nb(III) are able to scavenge peroxynitrite and to protect free l-tyrosine against peroxynitrite-mediated nitration. Innovation: Data here reported suggest an evolutionarily conserved function of Nbs related to their role as nitric oxide sensors and components of antioxidant systems. Conclusion: Human THAP4 may act as a sensing protein that couples the heme-based Nb(III) reactivity with gene transcription. Mt -Nb(III) seems to be part of the pool of proteins required to scavenge reactive nitrogen and oxygen species produced by the host during the immunity response.
Organizational Affiliation:
Dipartimento di Scienze, Università Roma Tre, Roma, Italy.