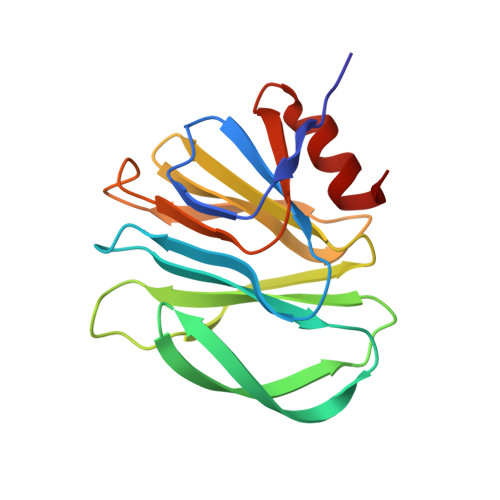
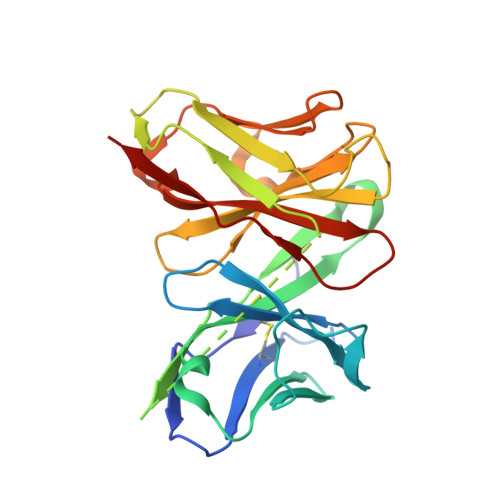
Human VP8* mAbs neutralize rotavirus selectively in human intestinal epithelial cells.
Feng, N., Hu, L., Ding, S., Sanyal, M., Zhao, B., Sankaran, B., Ramani, S., McNeal, M., Yasukawa, L.L., Song, Y., Prasad, B.V.V., Greenberg, H.B.(2019) J Clin Invest 130: 3839-3851
- PubMed: 31403468
- DOI: https://doi.org/10.1172/JCI128382
- Primary Citation of Related Structures:
6PCU - PubMed Abstract:
We previously generated 32 rotavirus-specific (RV-specific) recombinant monoclonal antibodies (mAbs) derived from B cells isolated from human intestinal resections. Twenty-four of these mAbs were specific for the VP8* fragment of RV VP4, and most (20 of 24) were non-neutralizing when tested in the conventional MA104 cell-based assay. We reexamined the ability of these mAbs to neutralize RVs in human intestinal epithelial cells including ileal enteroids and HT-29 cells. Most (18 of 20) of the "non-neutralizing" VP8* mAbs efficiently neutralized human RV in HT-29 cells or enteroids. Serum RV neutralization titers in adults and infants were significantly higher in HT-29 than MA104 cells and adsorption of these sera with recombinant VP8* lowered the neutralization titers in HT-29 but not MA104 cells. VP8* mAbs also protected suckling mice from diarrhea in an in vivo challenge model. X-ray crystallographic analysis of one VP8* mAb (mAb9) in complex with human RV VP8* revealed that the mAb interaction site was distinct from the human histo-blood group antigen binding site. Since MA104 cells are the most commonly used cell line to detect anti-RV neutralization activity, these findings suggest that prior vaccine and other studies of human RV neutralization responses may have underestimated the contribution of VP8* antibodies to the overall neutralization titer.
Organizational Affiliation:
Departments of Medicine and Microbiology and Immunology, School of Medicine, Stanford University, Stanford, California, USA.