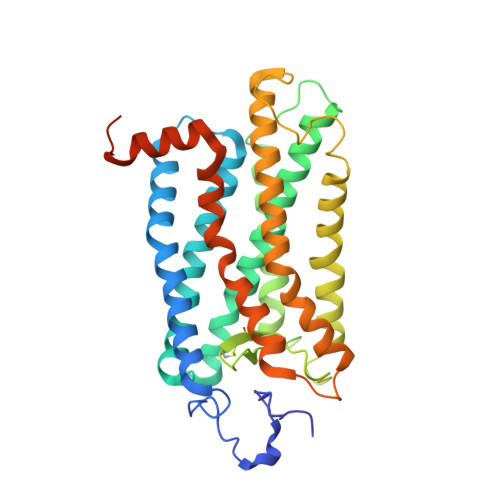
Cryo-EM structure of the native rhodopsin dimer in nanodiscs.
Zhao, D.Y., Poge, M., Morizumi, T., Gulati, S., Van Eps, N., Zhang, J., Miszta, P., Filipek, S., Mahamid, J., Plitzko, J.M., Baumeister, W., Ernst, O.P., Palczewski, K.(2019) J Biol Chem 294: 14215-14230
- PubMed: 31399513
- DOI: https://doi.org/10.1074/jbc.RA119.010089
- Primary Citation of Related Structures:
6OFJ - PubMed Abstract:
Imaging of rod photoreceptor outer-segment disc membranes by atomic force microscopy and cryo-electron tomography has revealed that the visual pigment rhodopsin, a prototypical class A G protein-coupled receptor (GPCR), can organize as rows of dimers. GPCR dimerization and oligomerization offer possibilities for allosteric regulation of GPCR activity, but the detailed structures and mechanism remain elusive. In this investigation, we made use of the high rhodopsin density in the native disc membranes and of a bifunctional cross-linker that preserves the native rhodopsin arrangement by covalently tethering rhodopsins via Lys residue side chains. We purified cross-linked rhodopsin dimers and reconstituted them into nanodiscs for cryo-EM analysis. We present cryo-EM structures of the cross-linked rhodopsin dimer as well as a rhodopsin dimer reconstituted into nanodiscs from purified monomers. We demonstrate the presence of a preferential 2-fold symmetrical dimerization interface mediated by transmembrane helix 1 and the cytoplasmic helix 8 of rhodopsin. We confirmed this dimer interface by double electron-electron resonance measurements of spin-labeled rhodopsin. We propose that this interface and the arrangement of two protomers is a prerequisite for the formation of the observed rows of dimers. We anticipate that the approach outlined here could be extended to other GPCRs or membrane receptors to better understand specific receptor dimerization mechanisms.
Organizational Affiliation:
Department of Biochemistry, University of Toronto, Toronto, Ontario M5S 1A8, Canada.