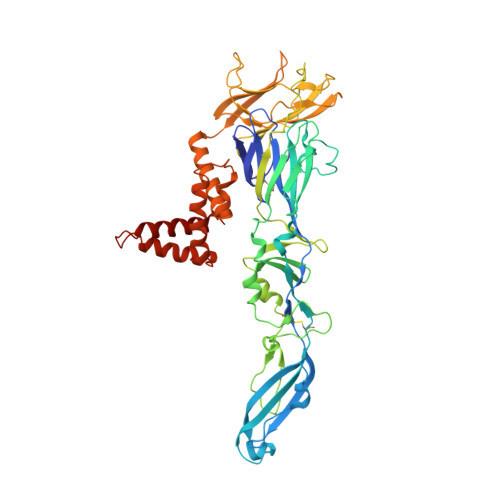
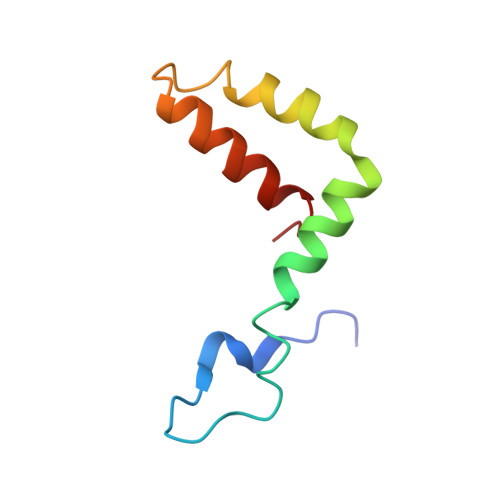
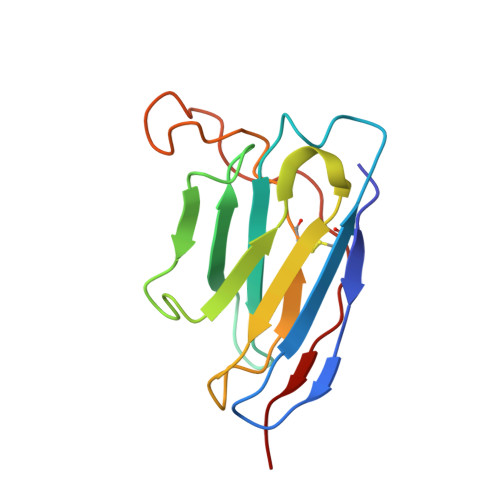
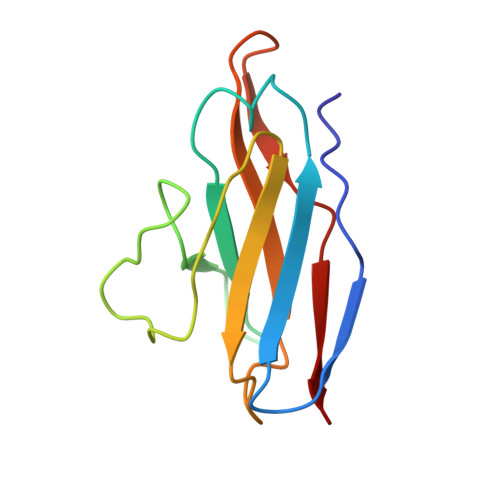
Structural basis of a potent human monoclonal antibody against Zika virus targeting a quaternary epitope.
Long, F., Doyle, M., Fernandez, E., Miller, A.S., Klose, T., Sevvana, M., Bryan, A., Davidson, E., Doranz, B.J., Kuhn, R.J., Diamond, M.S., Crowe Jr., J.E., Rossmann, M.G.(2019) Proc Natl Acad Sci U S A 116: 1591-1596
- PubMed: 30642974
- DOI: https://doi.org/10.1073/pnas.1815432116
- Primary Citation of Related Structures:
6MID - PubMed Abstract:
Zika virus (ZIKV) is a major human pathogen and member of the Flavivirus genus in the Flaviviridae family. In contrast to most other insect-transmitted flaviviruses, ZIKV also can be transmitted sexually and from mother to fetus in humans. During recent outbreaks, ZIKV infections have been linked to microcephaly, congenital disease, and Guillain-Barré syndrome. Neutralizing antibodies have potential as therapeutic agents. We report here a 4-Å-resolution cryo-electron microscopy structure of the ZIKV virion in complex with Fab fragments of the potently neutralizing human monoclonal antibody ZIKV-195. The footprint of the ZIKV-195 Fab fragment expands across two adjacent envelope (E) protein protomers. ZIKV neutralization by this antibody is presumably accomplished by cross-linking the E proteins, which likely prevents formation of E protein trimers required for fusion of the viral and cellular membranes. A single dose of ZIKV-195 administered 5 days after virus inoculation showed marked protection against lethality in a stringent mouse model of infection.
Organizational Affiliation:
Department of Biological Sciences, Purdue University, West Lafayette, IN 47907.