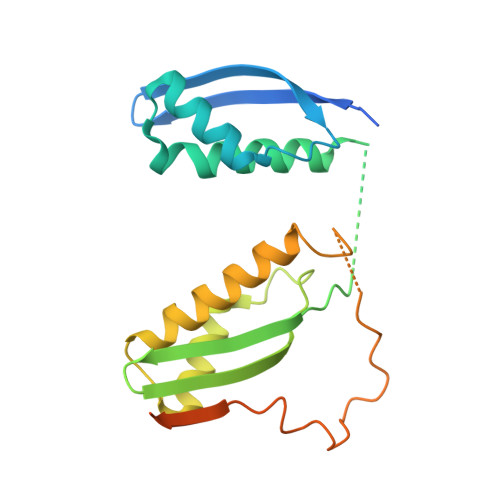
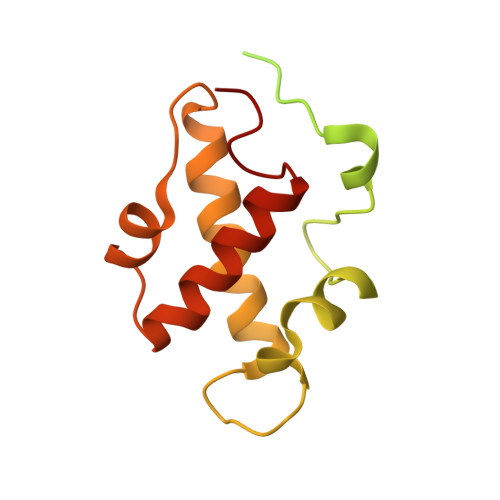
A heterotrimeric SMARCB1-SMARCC2 subcomplex is required for the assembly and tumor suppression function of the BAF chromatin-remodeling complex.
Chen, G., Zhou, H., Liu, B., Wang, Y., Zhao, J., Giancotti, F.G., Long, J.(2020) Cell Discov 6: 66-66
- PubMed: 33024572
- DOI: https://doi.org/10.1038/s41421-020-00196-4
- Primary Citation of Related Structures:
6KAG
Organizational Affiliation:
State Key Laboratory of Medicinal Chemical Biology and College of Life Sciences, Nankai University, 94 Weijin Road, Tianjin 300071, China.