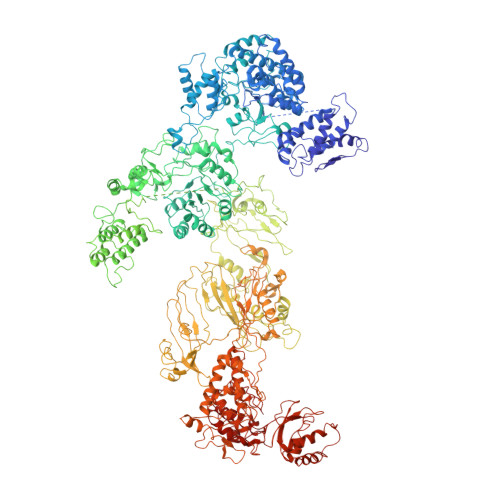
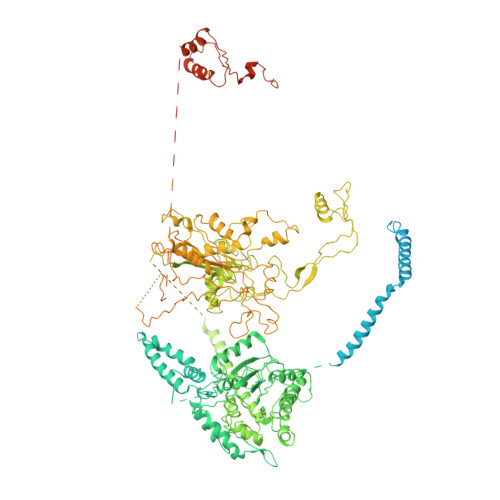
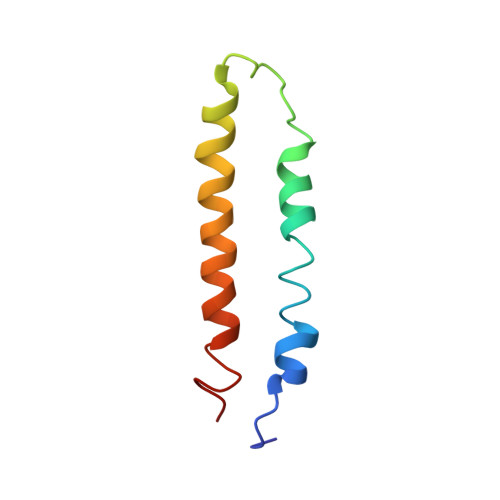
Modulation of fatty acid synthase by ATR checkpoint kinase Rad3.
Qiu, S., Liu, S., Zaoti, Z.F., Wang, X., Cai, G.(2019) J Mol Cell Biol 11: 1098-1100
- PubMed: 31509190
- DOI: https://doi.org/10.1093/jmcb/mjz096
- Primary Citation of Related Structures:
6JSH, 6JSI
Organizational Affiliation:
First Affiliated Hospital of USTC, School of Life Sciences, Hefei National Laboratory for Physical Sciences at Microscale, University of Science and Technology of China, Hefei 230027, China.