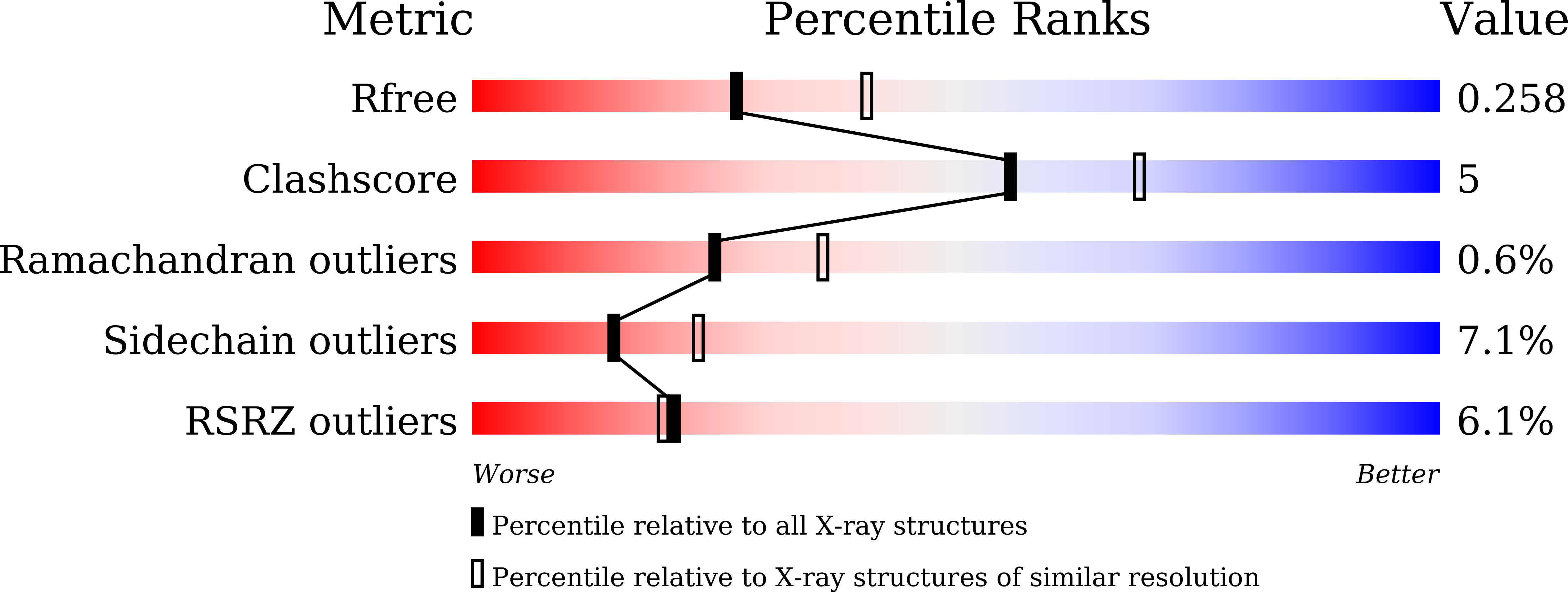
Reaction mechanism of the reverse reaction of African human trypanosomes glycerol kinase.
Balogun, E.O., Chishima, T., Ichinose, M., Inaoka, D.K., Kido, W., Ibrahim, B., de Koning, H., McKerrow, J.H., Watanabe, Y., Nozaki, T., Michels, P.A.M., Harada, S., Kita, K., Shiba, T.To be published.