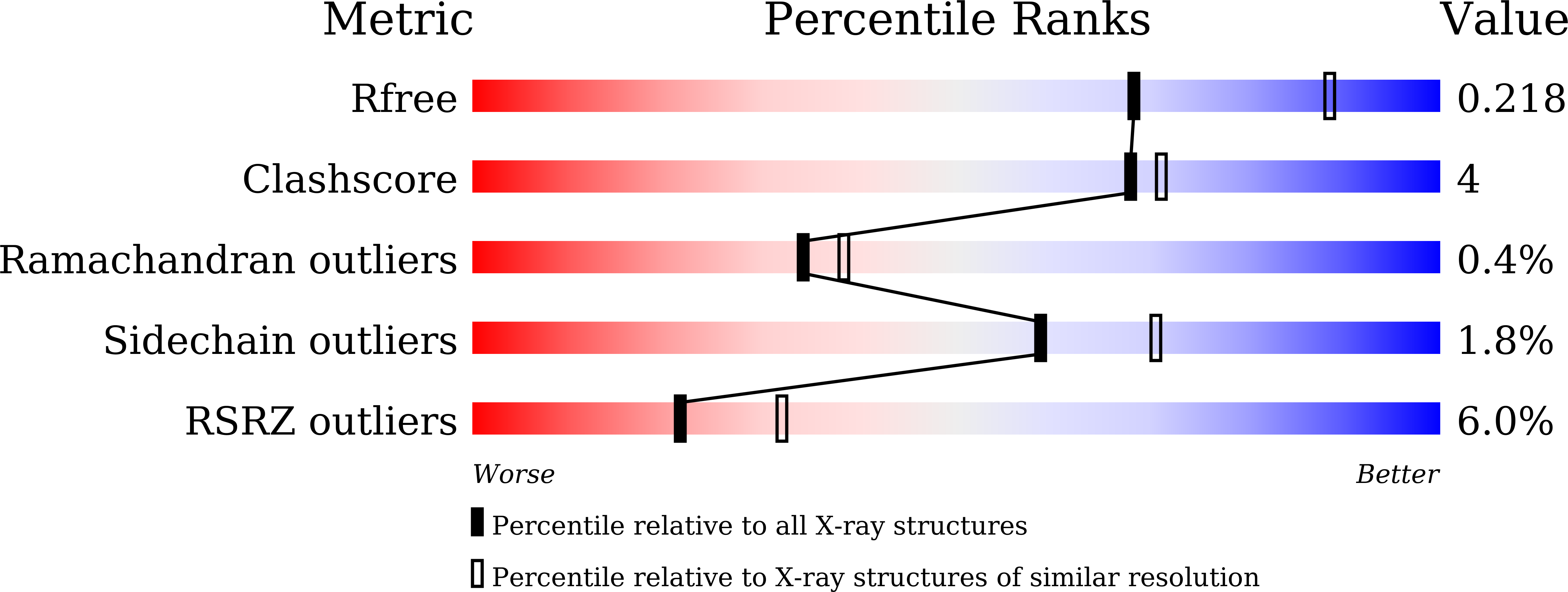
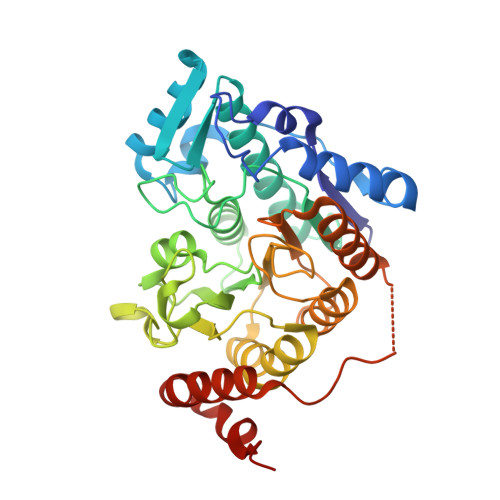
Structure of Arabidopsis HISTONE DEACETYLASE15.
Chen, C.Y., Tu, Y.T., Hsu, J.C., Hung, H.C., Liu, T.C., Lee, Y.H., Chou, C.C., Cheng, Y.S., Wu, K.(2020) Plant Physiol 184: 1585-1600
- PubMed: 32878973
- DOI: https://doi.org/10.1104/pp.20.00604
- Primary Citation of Related Structures:
6J6T - PubMed Abstract:
Mammalian histone deacetylases (HDACs) undergo phosphorylation to regulate their localization, activity, and function. However, little is known about the regulation of plant HDAC function and activity by phosphorylation. Here, we report the crystal structure of the Reduced Potassium Dependency3/Histone Deacetylase1 (RPD3/HDA1) type class II histone deacetylase HDA15 in Arabidopsis ( Arabidopsis thaliana ). The histone deacetylase domain of HDA15 (HDA15HD) assembles as tetrameric forms with each monomer composed of 12 α-helices and 9 β-sheets. The L1 loop and β2 sheet of HDA15HD are the essential interfaces for the tetramer formation. The N-terminal zinc finger domain enhances HDA15HD dimerization and increases its enzymatic activity. Furthermore, HDA15 can also be phosphorylated at Ser-448 and Ser-452 in etiolated seedlings. The HDA15 phosphorylation status determines its subnuclear localization and oligomerization. Phosphomimetics of HDA15 partially disrupt its oligomerization and cause loss of enzymatic activity and translocation from the nucleolus into nucleoplasm. Together, these data indicate that phosphorylation plays a critical role in regulating the structure and function of HDA15.
Organizational Affiliation:
Institute of Plant Biology, National Taiwan University, Taipei 10617, Taiwan.