Characterization of an SSB-dT25 complex: structural insights into the S-shaped ssDNA binding conformation.
Huang, Y.H., Chen, I.C., Huang, C.Y.(2019) RSC Adv 9: 40388-40396
Experimental Data Snapshot
wwPDB Validation 3D Report Full Report
(2019) RSC Adv 9: 40388-40396
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Single-stranded DNA-binding protein | 121 | Pseudomonas aeruginosa PAO1 | Mutation(s): 0 Gene Names: ssb, PA4232 | 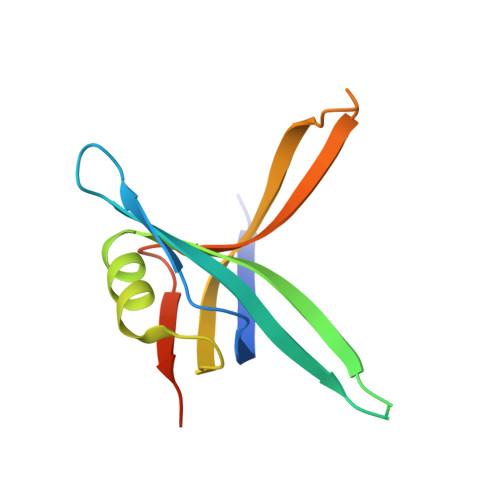 | |
UniProt | |||||
Find proteins for P40947 (Pseudomonas aeruginosa (strain ATCC 15692 / DSM 22644 / CIP 104116 / JCM 14847 / LMG 12228 / 1C / PRS 101 / PAO1)) Explore P40947 Go to UniProtKB: P40947 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P40947 | ||||
Sequence AnnotationsExpand | |||||
| |||||
Find similar nucleic acids by: Sequence | 3D Structure
Entity ID: 2 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Length | Organism | Image | |
| DNA (25-MER) | 25 | synthetic construct | 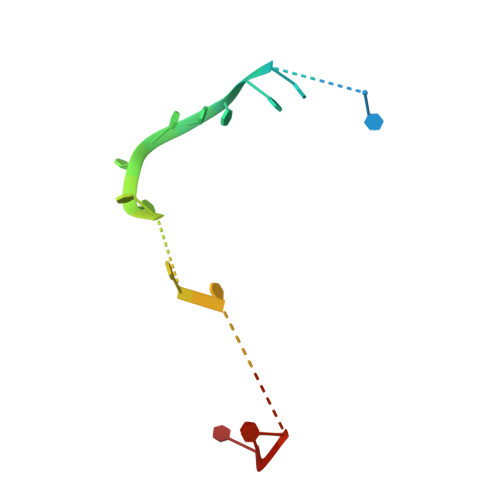 | ||
Sequence AnnotationsExpand | |||||
| |||||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 60.488 | α = 90 |
| b = 60.488 | β = 90 |
| c = 131.315 | γ = 120 |
| Software Name | Purpose |
|---|---|
| PHENIX | refinement |
| HKL-2000 | data reduction |
| HKL-2000 | data scaling |
| MOLREP | phasing |