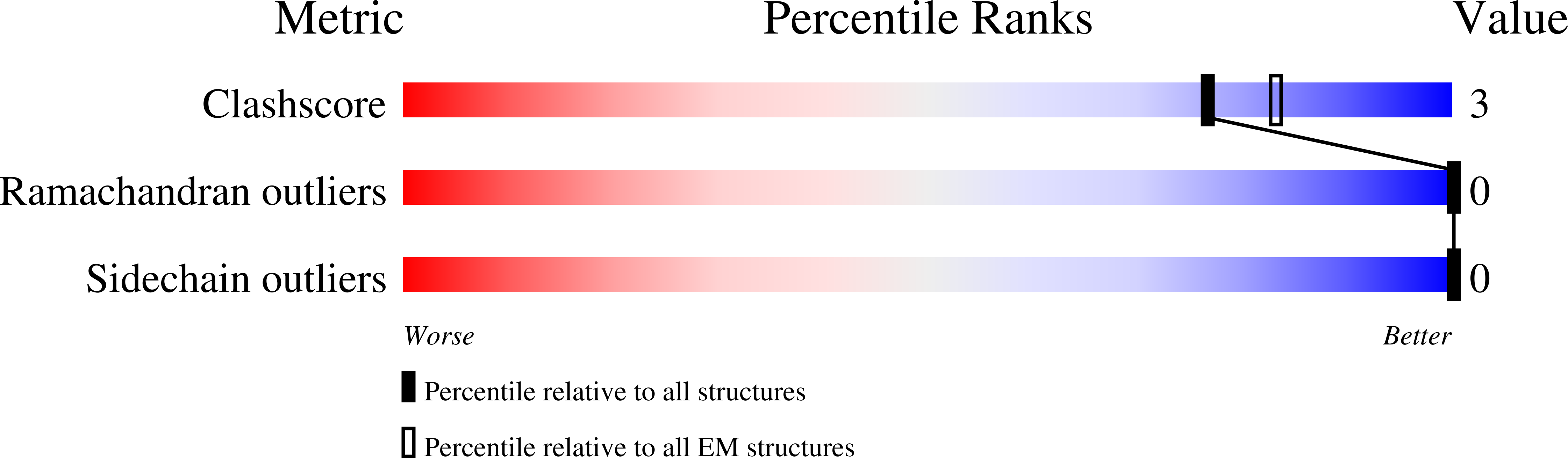MyD88 Death-Domain Oligomerization Determines Myddosome Architecture: Implications for Toll-like Receptor Signaling.
Moncrieffe, M.C., Bollschweiler, D., Li, B., Penczek, P.A., Hopkins, L., Bryant, C.E., Klenerman, D., Gay, N.J.(2020) Structure 28: 281-289.e3
- PubMed: 31995744
- DOI: https://doi.org/10.1016/j.str.2020.01.003
- Primary Citation of Related Structures:
6I3N - PubMed Abstract:
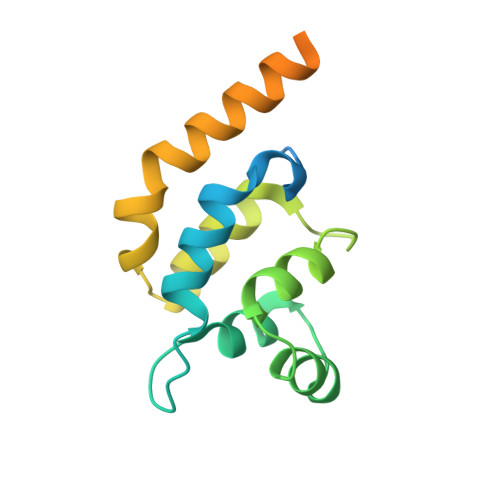
Toll-like receptors (TLRs) are pivotal in triggering the innate immune response to pathogen infection. Ligand binding induces receptor dimerization which facilitates the recruitment of other post-receptor signal transducers into a complex signalosome, the Myddosome. Central to this process is Myeloid differentiation primary response 88 (MyD88), which is required by almost all TLRs, and signaling is thought to proceed via the stepwise, sequential assembly of individual components. Here, we show that the death domains of human MyD88 spontaneously and reversibly associate to form helical filaments in vitro. A 3.1-Å cryoelectron microscopy structure reveals that the architecture of the filament is identical to that of the 6:4 MyD88-IRAK4-IRAK2 hetero-oligomeric Myddosome. Additionally, the death domain of IRAK4 interacts with the filaments to reconstitute the non-stoichiometric 6:4 MyD88-IRAK4 complex. Together, these data suggest that intracellularly, the MyD88 scaffold may be pre-formed and poised for recruitment of IRAKs on receptor activation and TIR engagement.
Organizational Affiliation:
Department of Biochemistry, University of Cambridge, Cambridge CB2 1GA, UK. Electronic address: mcm35@cam.ac.uk.