Rutinosidase from Aspergillus niger: crystal structure and insight into the enzymatic activity.
Pachl, P., Kapesova, J., Brynda, J., Biedermannova, L., Pelantova, H., Bojarova, P., Kren, V., Rezacova, P., Kotik, M.(2020) FEBS J 287: 3315-3327
- PubMed: 31943739
- DOI: https://doi.org/10.1111/febs.15208
- Primary Citation of Related Structures:
6I1A - PubMed Abstract:
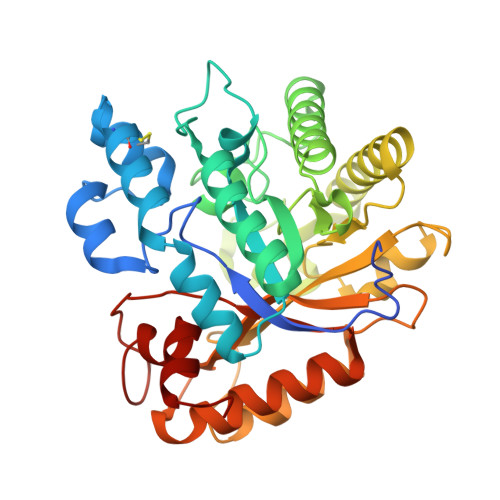
Rutinosidases (α-l-rhamnosyl-β-d-glucosidases) catalyze the cleavage of the glycosidic bond between the aglycone and the disaccharide rutinose (α-l-rhamnopyranosyl-(1→6)-β-d-glucopyranose) of specific flavonoid glycosides such as rutin (quercetin 3-O-rutinoside). Microbial rutinosidases are part of the rutin catabolic pathway, enabling the microorganism to utilize rutin and related plant phenolic glycosides. Here, we report the first three-dimensional structure of a rutinosidase determined at 1.27-Å resolution. The rutinosidase from Aspergillus niger K2 (AnRut), a member of glycoside hydrolase family GH-5, subfamily 23, was heterologously produced in Pichia pastoris. The X-ray structure of AnRut is represented by a distorted (β/α) 8 barrel fold with its closest structural homologue being an exo-β-(1,3)-glucanase from Candida albicans (CaExg). The catalytic site is located in a deep pocket with a striking structural similarity to CaExg. However, the entrance to the active site of AnRut has been found to be different from that of CaExg - a mostly unstructured section of ~ 40 residues present in CaExg is missing in AnRut, whereas an additional loop of 13 amino acids partially covers the active site of AnRut. NMR analysis of reaction products provided clear evidence for a retaining reaction mechanism of AnRut. Unexpectedly, quercetin 3-O-glucoside was found to be a better substrate than rutin, and thus, AnRut cannot be considered a typical diglycosidase. Mutational analysis of conserved active site residues in combination with in silico modeling allowed identification of essential interactions for enzyme activity and helped to reveal further details of substrate binding. The protein sequence of AnRut has been revised. DATABASES: The nucleotide sequence of the rutinosidase-encoding gene is available in the GenBank database under the accession number MN393234. Structural data are available in the PDB database under the accession number 6I1A. ENZYME: α-l-Rhamnosyl-β-d-glucosidase (EC 3.2.1.168).
Organizational Affiliation:
Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences, Prague, Czech Republic.