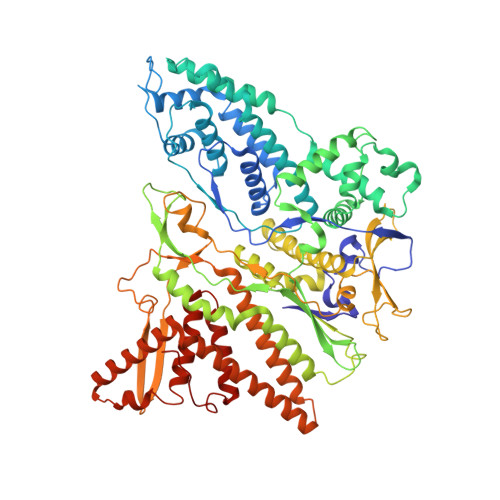
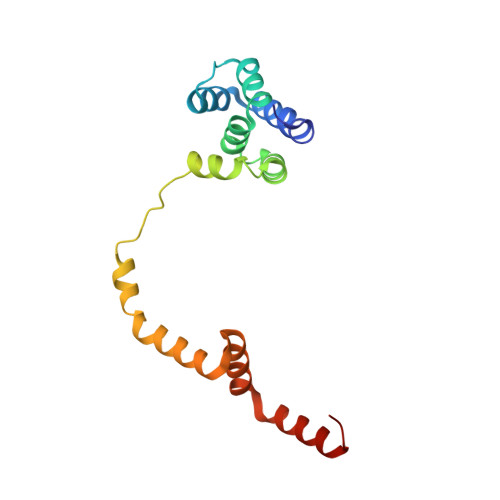
Multiple liquid crystalline geometries of highly compacted nucleic acid in a dsRNA virus.
Ilca, S.L., Sun, X., El Omari, K., Kotecha, A., de Haas, F., DiMaio, F., Grimes, J.M., Stuart, D.I., Poranen, M.M., Huiskonen, J.T.(2019) Nature 570: 252-256
- PubMed: 31142835
- DOI: https://doi.org/10.1038/s41586-019-1229-9
- Primary Citation of Related Structures:
6HY0 - PubMed Abstract:
Characterizing the genome of mature virions is pivotal to understanding the highly dynamic processes of virus assembly and infection. Owing to the different cellular fates of DNA and RNA, the life cycles of double-stranded (ds)DNA and dsRNA viruses are dissimilar. In terms of nucleic acid packing, dsDNA viruses, which lack genome segmentation and intra-capsid transcriptional machinery, predominantly display single-spooled genome organizations 1-8 . Because the release of dsRNA into the cytoplasm triggers host defence mechanisms 9 , dsRNA viruses retain their genomes within a core particle that contains the enzymes required for RNA replication and transcription 10-12 . The genomes of dsRNA viruses vary greatly in the degree of segmentation. In members of the Reoviridae family, genomes consist of 10-12 segments and exhibit a non-spooled arrangement mediated by RNA-dependent RNA polymerases 11-14 . However, whether this arrangement is a general feature of dsRNA viruses remains unknown. Here, using cryo-electron microscopy to resolve the dsRNA genome structure of the tri-segmented bacteriophage ɸ6 of the Cystoviridae family, we show that dsRNA viruses can adopt a dsDNA-like single-spooled genome organization. We find that in this group of viruses, RNA-dependent RNA polymerases do not direct genome ordering, and the dsRNA can adopt multiple conformations. We build a model that encompasses 90% of the genome, and use this to quantify variation in the packing density and to characterize the different liquid crystalline geometries that are exhibited by the tightly compacted nucleic acid. Our results demonstrate that the canonical model for the packing of dsDNA can be extended to dsRNA viruses.
Organizational Affiliation:
Division of Structural Biology, Wellcome Centre for Human Genetics, University of Oxford, Oxford, UK.