Molecular Recognition of a Thomsen-Friedenreich Antigen Mimetic Targeting Human Galectin-3.
Santarsia, S., Grosso, A.S., Trovao, F., Jimenez-Barbero, J., Carvalho, A.L., Nativi, C., Marcelo, F.(2018) ChemMedChem 13: 2030-2036
- PubMed: 30094951
- DOI: https://doi.org/10.1002/cmdc.201800525
- Primary Citation of Related Structures:
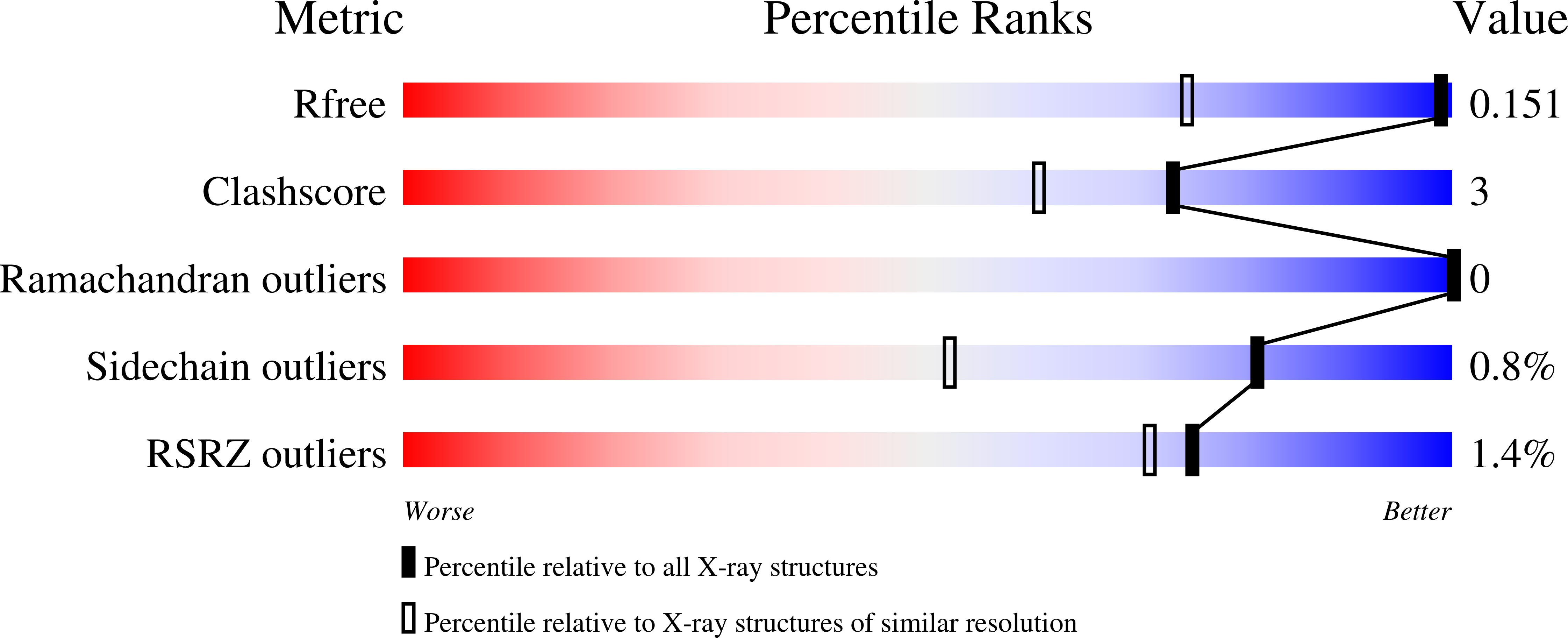
6G0V - PubMed Abstract:
Overexpression of the Thomsen-Friedenreich (TF) antigen in cell membrane proteins occurs in 90 % of adenocarcinomas. Additionally, the binding of the TF antigen to human galectin-3 (Gal-3), also frequently overexpressed in malignancy, promotes cancer progression and metastasis. In this context, structures that interfere with this specific interaction have the potential to prevent cancer metastasis. A multidisciplinary approach combining the optimized synthesis of a TF antigen mimetic with NMR, X-ray crystallography methods, and isothermal titration calorimetry assays was used to unravel the molecular structural details that govern the Gal-3/TF mimetic interaction. The TF mimetic has a binding affinity for Gal-3 similar to that of the TF natural antigen and retains the binding epitope and bioactive conformation observed for the native antigen. Furthermore, from a thermodynamic perspective, a decrease in the enthalpic contribution was observed for the Gal-3/TF mimetic complex; however, this behavior is compensated by a favorable gain in entropy. From a structural perspective, these results establish our TF mimetic as a scaffold to design multivalent solutions to potentially interfere with Gal-3 aberrant interactions and for likely use in hampering Gal-3-mediated cancer cell adhesion and metastasis.
Organizational Affiliation:
Department of Chemistry Ugo Schiff, University of Florence, Via della Lastruccia, 13-50019, Sesto Fiorentino, Italy.