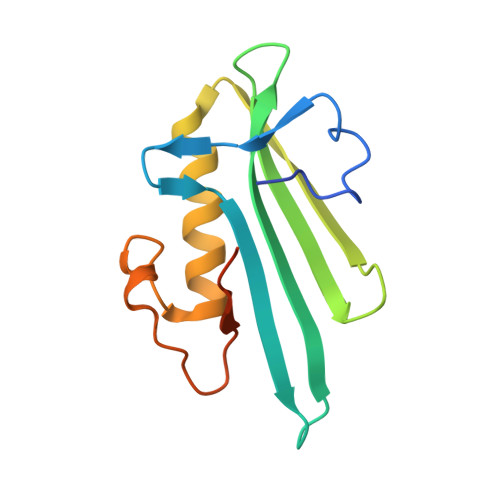
Structural basis for DNA break recognition by ARTD2/PARP2.
Obaji, E., Haikarainen, T., Lehtio, L.(2018) Nucleic Acids Res 46: 12154-12165
- PubMed: 30321391
- DOI: https://doi.org/10.1093/nar/gky927
- Primary Citation of Related Structures:
6F1K, 6F5B, 6F5F - PubMed Abstract:
Human ARTD2 (or PARP2) is an ADP-ribosyltransferase, which is catalytically activated by binding to damaged DNA. ARTD2 subsequently ADP-ribosylates itself and other proteins, initiating a cascade of events leading to DNA repair. In contrast to ARTD1, the founding member of the enzyme family, ARTD2 does not have specialized zinc-fingers for detecting DNA damage. The domain organization of ARTD2 includes disordered N-terminus, WGR and catalytic domains. However, the N-terminus of ARTD2 is not strictly required for the DNA dependent activity. While it is known that ARTD2 requires the WGR domain for efficient DNA binding and subsequent catalytic activation, the mechanism of DNA damage detection and subsequent catalytic activation are not completely understood. Here, we report crystal structures of ARTD2 WGR domain bound to double-strand break mimicking DNA oligonucleotides. Notably, the crystal structures revealed DNA binding mode of ARTD2 involving DNA end to end interaction. Structures demonstrate how ARTD2 recognizes nicked DNA, how it interacts with the 5'-phosphate group, and how it can mediate joining of DNA ends in vitro. Extensive mutagenesis of the ARTD2-DNA interface combined with activity, binding, and stoichiometry measurements demonstrate that the WGR domain is the key for DNA break detection.
Organizational Affiliation:
Faculty of Biochemistry and Molecular Medicine and Biocenter Oulu, University of Oulu, Oulu, Finland.