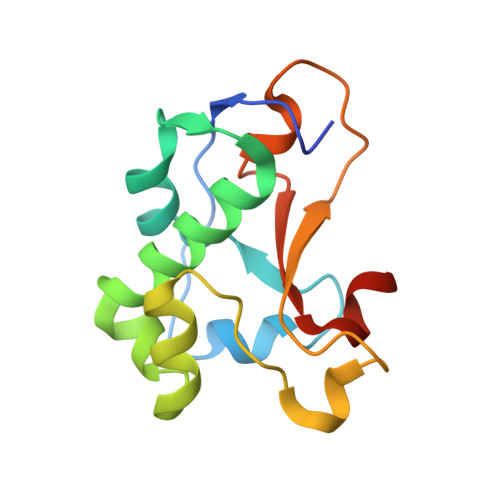
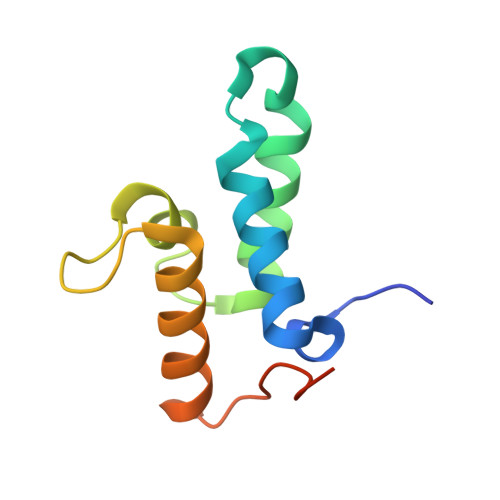
Ultrahigh specificity in a network of computationally designed protein-interaction pairs.
Netzer, R., Listov, D., Lipsh, R., Dym, O., Albeck, S., Knop, O., Kleanthous, C., Fleishman, S.J.(2018) Nat Commun 9: 5286-5286
- PubMed: 30538236
- DOI: https://doi.org/10.1038/s41467-018-07722-9
- Primary Citation of Related Structures:
6ER6, 6ERE - PubMed Abstract:
Protein networks in all organisms comprise homologous interacting pairs. In these networks, some proteins are specific, interacting with one or a few binding partners, whereas others are multispecific and bind a range of targets. We describe an algorithm that starts from an interacting pair and designs dozens of new pairs with diverse backbone conformations at the binding site as well as new binding orientations and sequences. Applied to a high-affinity bacterial pair, the algorithm results in 18 new ones, with cognate affinities from pico- to micromolar. Three pairs exhibit 3-5 orders of magnitude switch in specificity relative to the wild type, whereas others are multispecific, collectively forming a protein-interaction network. Crystallographic analysis confirms design accuracy, including in new backbones and polar interactions. Preorganized polar interaction networks are responsible for high specificity, thus defining design principles that can be applied to program synthetic cellular interaction networks of desired affinity and specificity.
Organizational Affiliation:
Department of Biomolecular Sciences, Weizmann Institute of Science, 7610001, Rehovot, Israel.