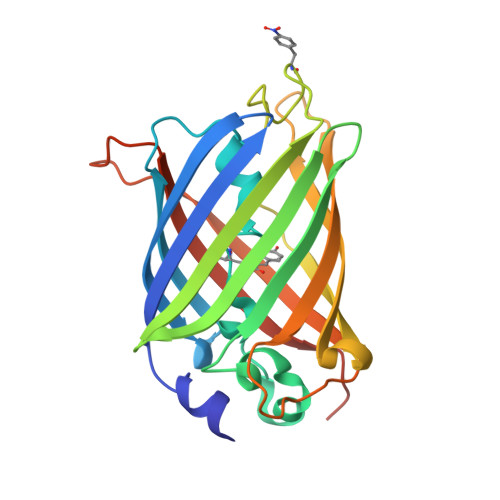
Crystal structures of green fluorescent protein with the unnatural amino acid 4-nitro-L-phenylalanine.
Maurici, N., Savidge, N., Lee, B.U., Brewer, S.H., Phillips-Piro, C.M.(2018) Acta Crystallogr F Struct Biol Commun 74: 650-655
- PubMed: 30279317
- DOI: https://doi.org/10.1107/S2053230X1801169X
- Primary Citation of Related Structures:
6DQ0, 6DQ1 - PubMed Abstract:
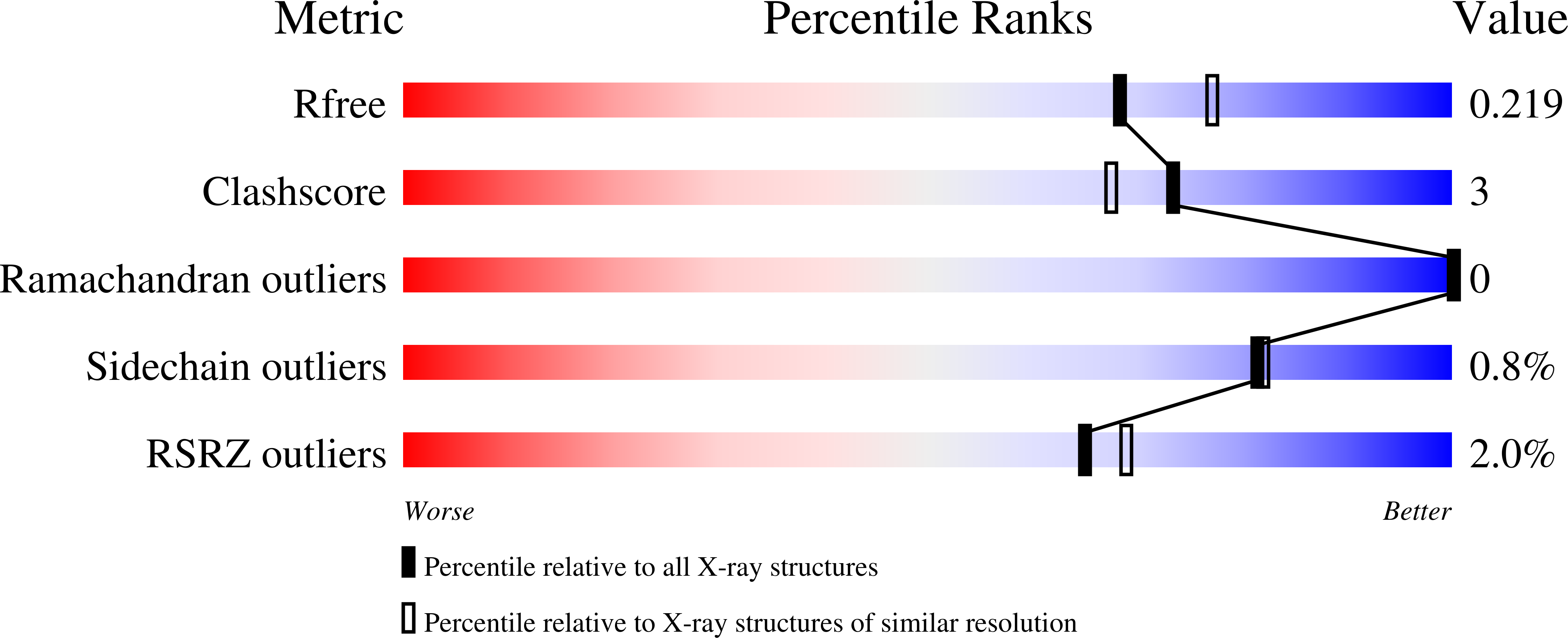
The X-ray crystal structures of two superfolder green fluorescent protein (sfGFP) constructs containing a genetically incorporated spectroscopic reporter unnatural amino acid, 4-nitro-L-phenylalanine (pNO 2 F), at two unique sites in the protein have been determined. Amber codon-suppression methodology was used to site-specifically incorporate pNO 2 F at a solvent-accessible (Asp133) and a partially buried (Asn149) site in sfGFP. The Asp133pNO 2 F sfGFP construct crystallized with two molecules per asymmetric unit in space group P3 2 21 and the crystal structure was refined to 2.05 Å resolution. Crystals of Asn149pNO 2 F sfGFP contained one molecule of sfGFP per asymmetric unit in space group P4 1 22 and the structure was refined to 1.60 Å resolution. The alignment of Asp133pNO 2 F or Asn149pNO 2 F sfGFP with wild-type sfGFP resulted in small root-mean-square deviations, illustrating that these residues do not significantly alter the protein structure and supporting the use of pNO 2 F as an effective spectroscopic reporter of local protein structure and dynamics.
Organizational Affiliation:
Department of Chemistry, Franklin and Marshall College, PO Box 3003, Lancaster, PA 17604, USA.