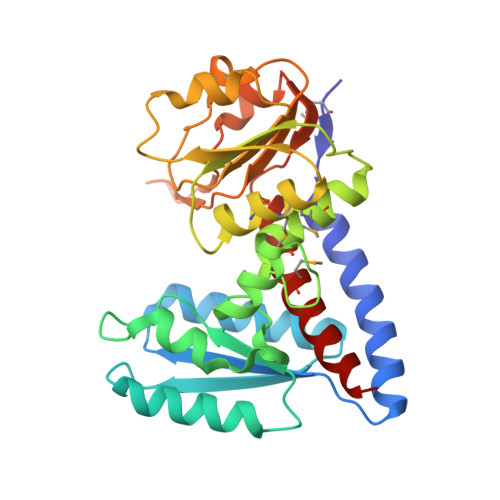
Crystal Structure of Bifunctional Enzyme FolD-Methylenetetrahydrofolate Dehydrogenase/Cyclohydrolase from Campylobacter jejuni
Kim, Y., Makowska-Grzyska, M., Zhang, R., Peterson, S.N., Joachimiak, A., Center for Structural Genomics of Infectious Diseases (CSGID)To be published.