Acyldepsipeptide Analogs Dysregulate Human Mitochondrial ClpP Protease Activity and Cause Apoptotic Cell Death.
Wong, K.S., Mabanglo, M.F., Seraphim, T.V., Mollica, A., Mao, Y.Q., Rizzolo, K., Leung, E., Moutaoufik, M.T., Hoell, L., Phanse, S., Goodreid, J., Barbosa, L.R.S., Ramos, C.H.I., Babu, M., Mennella, V., Batey, R.A., Schimmer, A.D., Houry, W.A.(2018) Cell Chem Biol 25: 1017-1030.e9
- PubMed: 30126533
- DOI: https://doi.org/10.1016/j.chembiol.2018.05.014
- Primary Citation of Related Structures:
6BBA - PubMed Abstract:
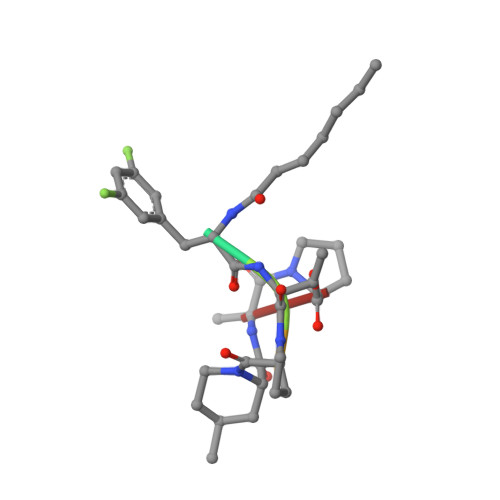
Acyldepsipeptides (ADEPs) are potential antibiotics that dysregulate the activity of the highly conserved tetradecameric bacterial ClpP protease, leading to bacterial cell death. Here, we identified ADEP analogs that are potent dysregulators of the human mitochondrial ClpP (HsClpP). These ADEPs interact tightly with HsClpP, causing the protease to non-specifically degrade model substrates. Dysregulation of HsClpP activity by ADEP was found to induce cytotoxic effects via activation of the intrinsic, caspase-dependent apoptosis. ADEP-HsClpP co-crystal structure was solved for one of the analogs revealing a highly complementary binding interface formed by two HsClpP neighboring subunits but, unexpectedly, with HsClpP in the compact conformation. Given that HsClpP is highly expressed in multiple cancers and has important roles in cell metastasis, our findings suggest a therapeutic potential for ADEPs in cancer treatment.
Organizational Affiliation:
Department of Biochemistry, University of Toronto, 661 University Avenue, MaRS Centre, West Tower, Room 1612, Toronto, ON M5G 1M1, Canada.