Effects of Single alpha-to-beta Residue Replacements on Recognition of an Extended Segment in a Viral Fusion Protein.
Outlaw, V.K., Kreitler, D.F., Stelitano, D., Porotto, M., Moscona, A., Gellman, S.H.(2020) ACS Infect Dis 6: 2017-2022
- PubMed: 32692914
- DOI: https://doi.org/10.1021/acsinfecdis.0c00385
- Primary Citation of Related Structures:
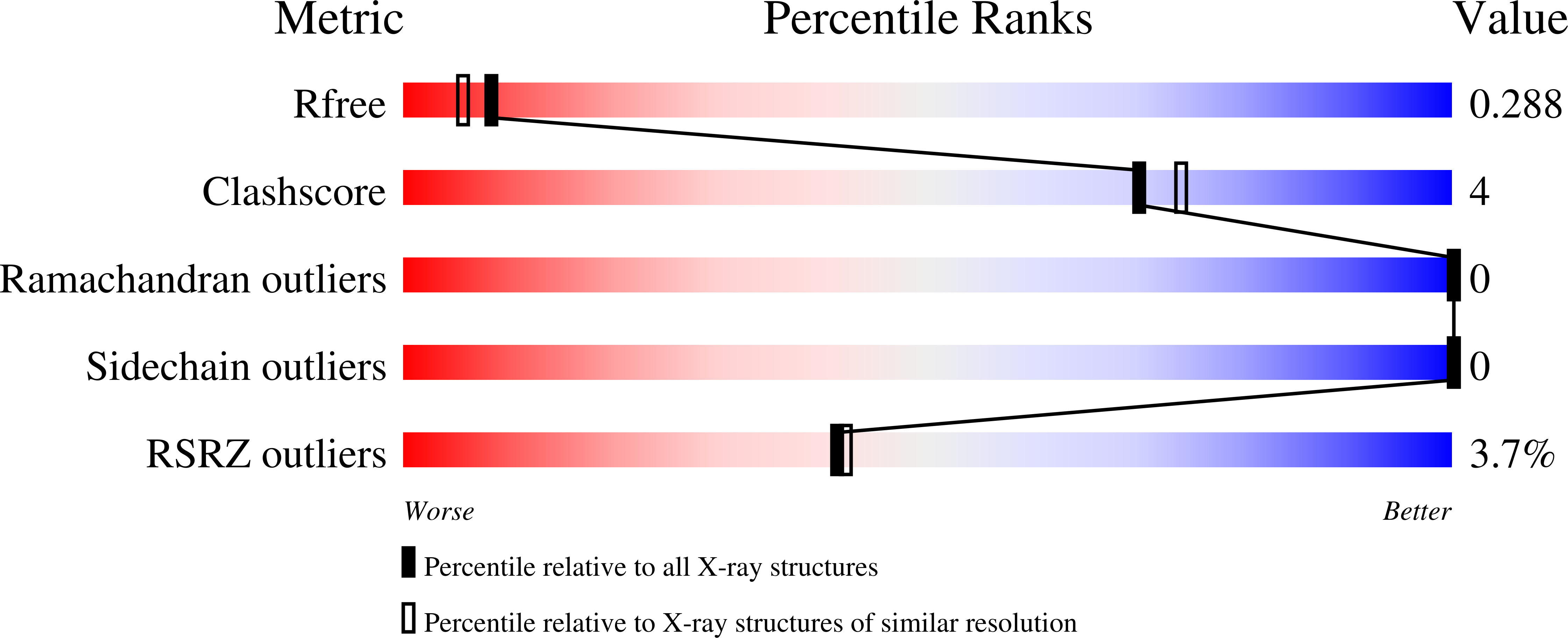
6PRL, 6PYQ, 6PZ6, 6V3V, 6VAS - PubMed Abstract:
Partial replacement of α-amino acid residues with β-amino acid residues has been established as a strategy for preserving target-engagement by helix-forming polypeptides while altering other properties. The impact of β-residue incorporation within polypeptides that adopt less regular conformations, however, has received less attention. The C-terminal heptad repeat (HRC) domains of fusion glycoproteins from pathogenic paramyxoviruses contain a segment that must adopt an extended conformation in order to coassemble with the N-terminal heptad repeat (HRN) domain in the postfusion state and drive a merger of the viral envelope with a target cell membrane. Here, we examine the impact of single α-to-β substitutions within this extended N-terminal segment of an engineered HRC peptide designated VIQKI. Stabilities of hexameric coassemblies formed with the native human parainfluenza virus 3 (HPIV3) HRN have been evaluated, the structures of five coassemblies have been determined, and antiviral efficacies have been measured. Many sites within the extended segment show functional tolerance of α-to-β substitution. These results offer a basis for future development of paramyxovirus infection inhibitors with novel biological activity profiles, possibly including resistance to proteolysis.
Organizational Affiliation:
Department of Chemistry, University of Wisconsin, Madison, Wisconsin 53706, United States.