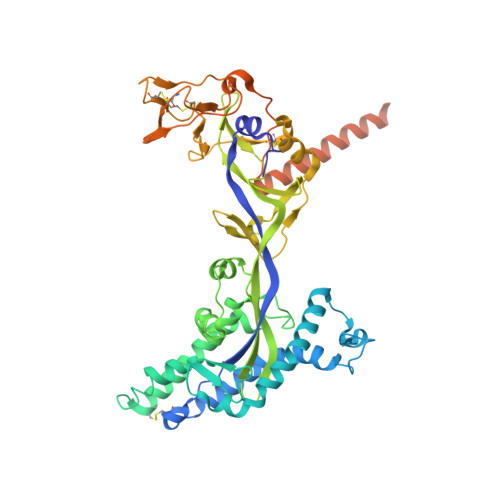
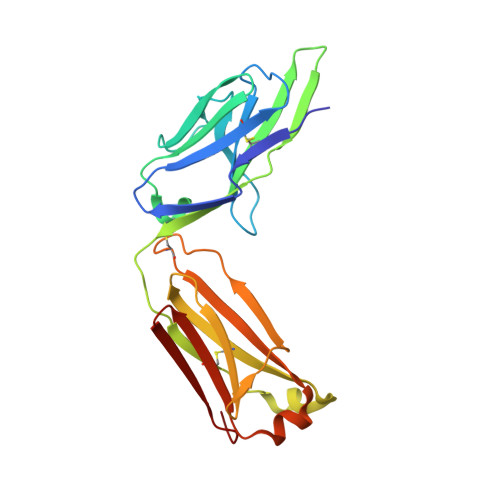
A structural basis for antibody-mediated neutralization of Nipah virus reveals a site of vulnerability at the fusion glycoprotein apex.
Avanzato, V.A., Oguntuyo, K.Y., Escalera-Zamudio, M., Gutierrez, B., Golden, M., Kosakovsky Pond, S.L., Pryce, R., Walter, T.S., Seow, J., Doores, K.J., Pybus, O.G., Munster, V.J., Lee, B., Bowden, T.A.(2019) Proc Natl Acad Sci U S A 116: 25057-25067
- PubMed: 31767754
- DOI: https://doi.org/10.1073/pnas.1912503116
- Primary Citation of Related Structures:
6T3F - PubMed Abstract:
Nipah virus (NiV) is a highly pathogenic paramyxovirus that causes frequent outbreaks of severe neurologic and respiratory disease in humans with high case fatality rates. The 2 glycoproteins displayed on the surface of the virus, NiV-G and NiV-F, mediate host-cell attachment and membrane fusion, respectively, and are targets of the host antibody response. Here, we provide a molecular basis for neutralization of NiV through antibody-mediated targeting of NiV-F. Structural characterization of a neutralizing antibody (nAb) in complex with trimeric prefusion NiV-F reveals an epitope at the membrane-distal domain III (DIII) of the molecule, a region that undergoes substantial refolding during host-cell entry. The epitope of this monoclonal antibody (mAb66) is primarily protein-specific and we observe that glycosylation at the periphery of the interface likely does not inhibit mAb66 binding to NiV-F. Further characterization reveals that a Hendra virus-F-specific nAb (mAb36) and many antibodies in an antihenipavirus-F polyclonal antibody mixture (pAb835) also target this region of the molecule. Integrated with previously reported paramyxovirus F-nAb structures, these data support a model whereby the membrane-distal region of the F protein is targeted by the antibody-mediated immune response across henipaviruses. Notably, our domain-specific sequence analysis reveals no evidence of selective pressure at this region of the molecule, suggestive that functional constraints prevent immune-driven sequence variation. Combined, our data reveal the membrane-distal region of NiV-F as a site of vulnerability on the NiV surface.
Organizational Affiliation:
Division of Structural Biology, Wellcome Center for Human Genetics, University of Oxford, OX3 7BN Oxford, United Kingdom.