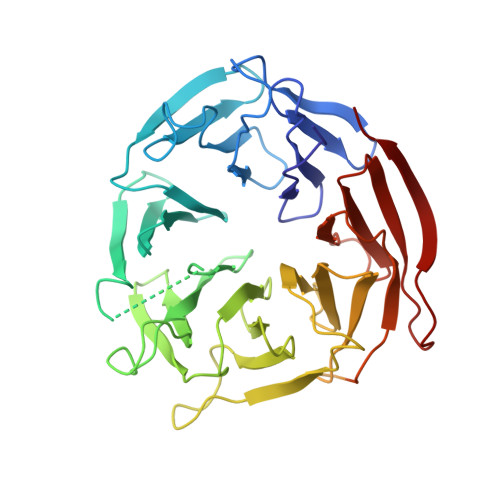
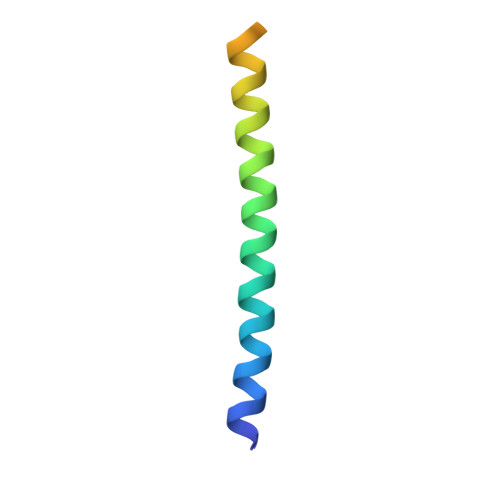
Atg21 organizes Atg8 lipidation at the contact of the vacuole with the phagophore.
Munzel, L., Neumann, P., Otto, F.B., Krick, R., Metje-Sprink, J., Kroppen, B., Karedla, N., Enderlein, J., Meinecke, M., Ficner, R., Thumm, M.(2020) Autophagy : 1-21
- PubMed: 32515645
- DOI: https://doi.org/10.1080/15548627.2020.1766332
- Primary Citation of Related Structures:
6RGO - PubMed Abstract:
Coupling of Atg8 to phosphatidylethanolamine is crucial for the expansion of the crescent-shaped phagophore during cargo engulfment. Atg21, a PtdIns3P-binding beta-propeller protein, scaffolds Atg8 and its E3-like complex Atg12-Atg5-Atg16 during lipidation. The crystal structure of Atg21, in complex with the Atg16 coiled-coil domain, showed its binding at the bottom side of the Atg21 beta-propeller. Our structure allowed detailed analyses of the complex formation of Atg21 with Atg16 and uncovered the orientation of the Atg16 coiled-coil domain with respect to the membrane. We further found that Atg21 was restricted to the phagophore edge, near the vacuole, known as the vacuole isolation membrane contact site (VICS). We identified a specialized vacuolar subdomain at the VICS, typical of organellar contact sites, where the membrane protein Vph1 was excluded, while Vac8 was concentrated. Furthermore, Vac8 was required for VICS formation. Our results support a specialized organellar contact involved in controlling phagophore elongation. Abbreviations : FCCS: fluorescence cross correlation spectroscopy; NVJ: nucleus-vacuole junction; PAS: phagophore assembly site; PE: phosphatidylethanolamine; PROPPIN: beta-propeller that binds phosphoinositides; PtdIns3P: phosphatidylinositol- 3-phosphate; VICS: vacuole isolation membrane contact site.
Organizational Affiliation:
Department of Cellular Biochemistry, University Medicine, Goettingen, Germany.