A dynamic charge-charge interaction modulates PP2A:B56 substrate recruitment.
Wang, X., Garvanska, D.H., Nasa, I., Ueki, Y., Zhang, G., Kettenbach, A.N., Peti, W., Nilsson, J., Page, R.(2020) Elife 9
- PubMed: 32195664
- DOI: https://doi.org/10.7554/eLife.55966
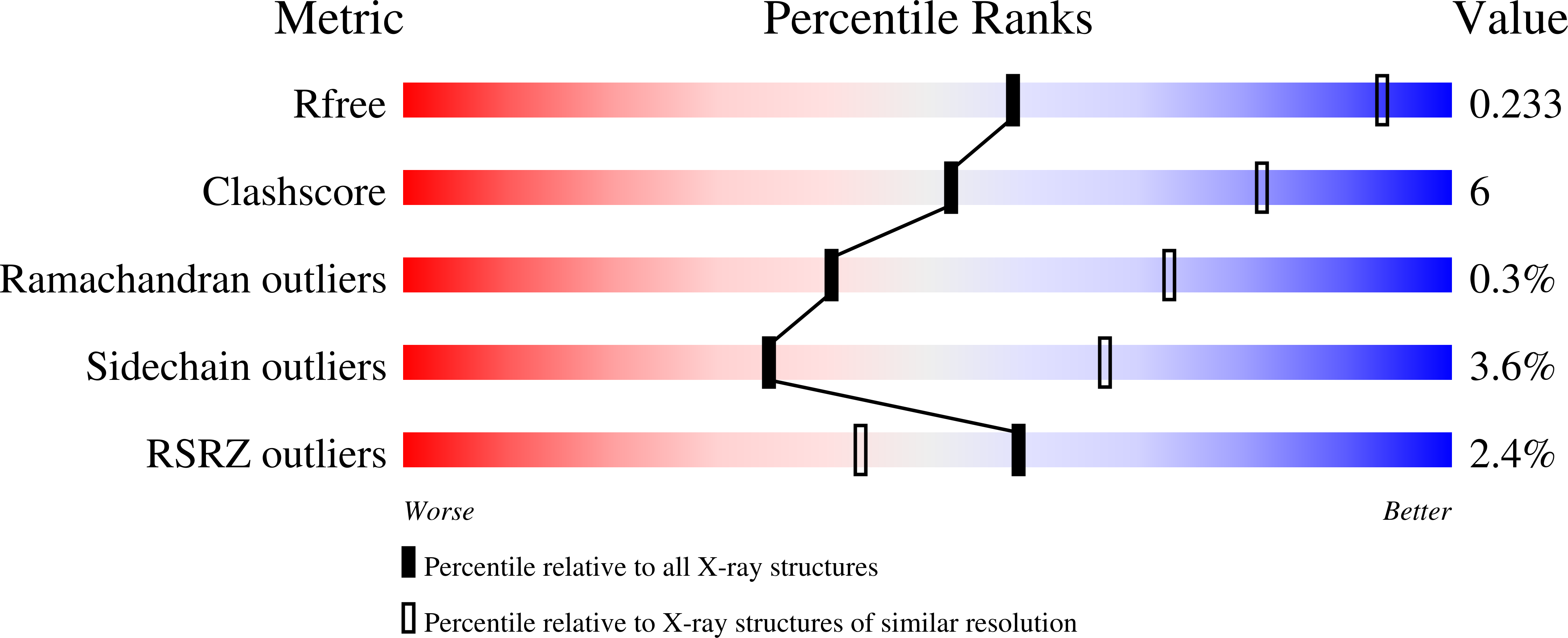
- Primary Citation of Related Structures:
6OYL, 6VRO - PubMed Abstract:
The recruitment of substrates by the ser/thr protein phosphatase 2A (PP2A) is poorly understood, limiting our understanding of PP2A-regulated signaling. Recently, the first PP2A:B56 consensus binding motif, LxxIxE, was identified. However, most validated LxxIxE motifs bind PP2A:B56 with micromolar affinities, suggesting that additional motifs exist to enhance PP2A:B56 binding. Here, we report the requirement of a positively charged motif in a subset of PP2A:B56 interactors, including KIF4A, to facilitate B56 binding via dynamic, electrostatic interactions. Using molecular and cellular experiments, we show that a conserved, negatively charged groove on B56 mediates dynamic binding. We also discovered that this positively charged motif, in addition to facilitating KIF4A dephosphorylation, is essential for condensin I binding, a function distinct and exclusive from PP2A-B56 binding. Together, these results reveal how dynamic, charge-charge interactions fine-tune the interactions mediated by specific motifs, providing a new framework for understanding how PP2A regulation drives cellular signaling.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of Arizona, Tucson, United States.