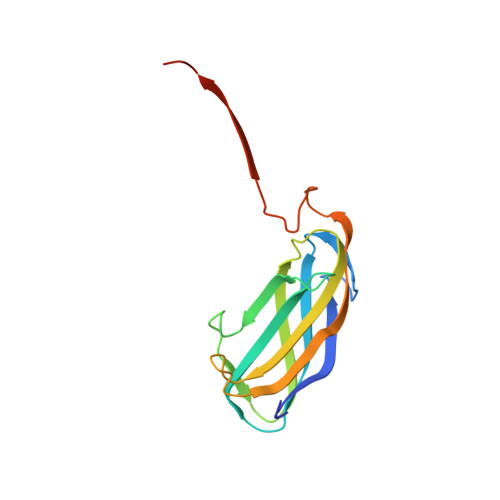
Structure and Ligand-Binding Properties of the O Antigen ABC Transporter Carbohydrate-Binding Domain.
Bi, Y., Zimmer, J.(2020) Structure 28: 252
- PubMed: 31879128
- DOI: https://doi.org/10.1016/j.str.2019.11.020
- Primary Citation of Related Structures:
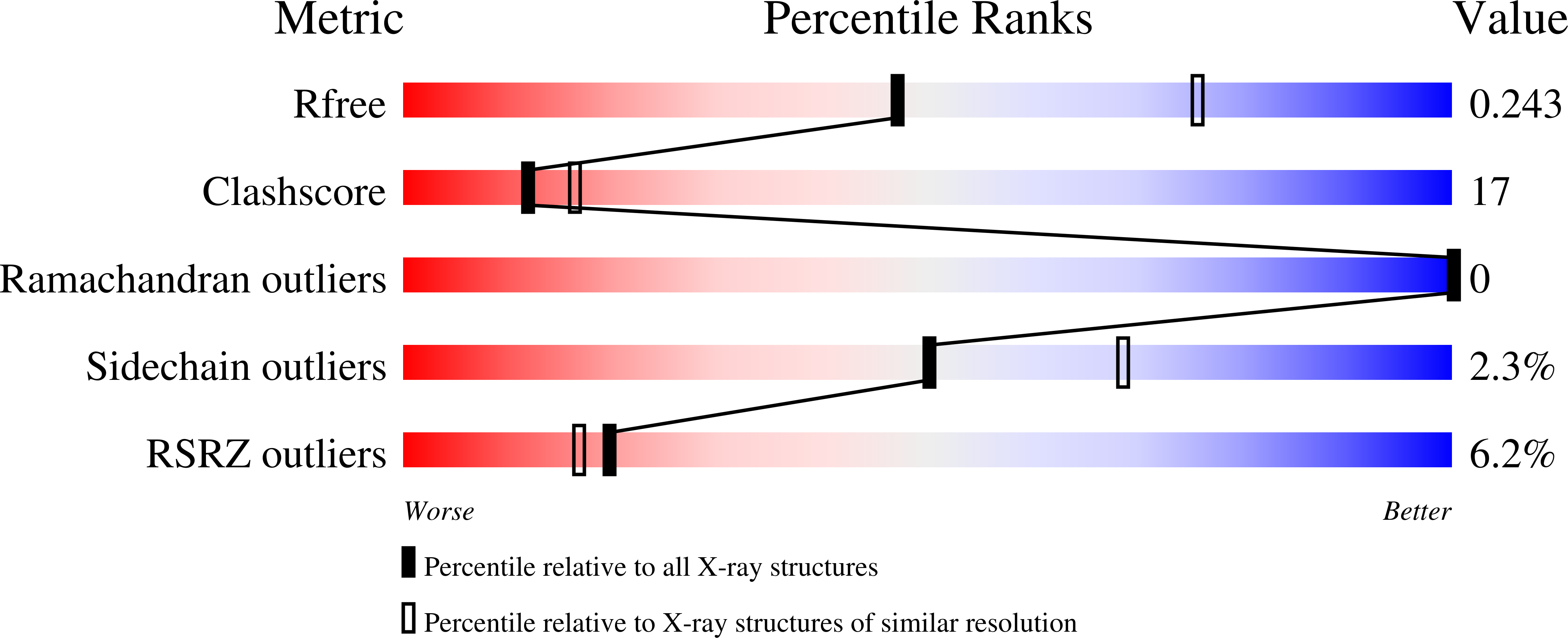
6O14 - PubMed Abstract:
A hallmark of Gram-negative bacteria is an asymmetric outer membrane containing lipopolysaccharides (LPSs) in the extracellular leaflet. LPS molecules consist of lipid A, which is connected to the inner and outer core oligosaccharides. This LPS core structure is extended in the periplasm by the O antigen, a variable and serotype-defining polysaccharide. In the ABC transporter-dependent LPS biosynthesis pathway, the WzmWzt transporter secretes the complete O antigen across the inner membrane for ligation to the LPS core. In some O antigen transporters, the nucleotide-binding domain of Wzt is fused C-terminally to a carbohydrate-binding domain (CBD) that interacts with the O antigen chain. Here, we present the crystal structure of the Aquifex aeolicus CBD that reveals a conserved flat and a variable twisted jelly-roll surface. The CBD dimer is stabilized by mutual β strand exchange. Microbial glycan array binding studies with the isolated CBD provide insights into its interaction with complex carbohydrates.
Organizational Affiliation:
School of Medicine, Department of Molecular Physiology and Biological Physics, University of Virginia, Charlottesville, VA 22903, USA.