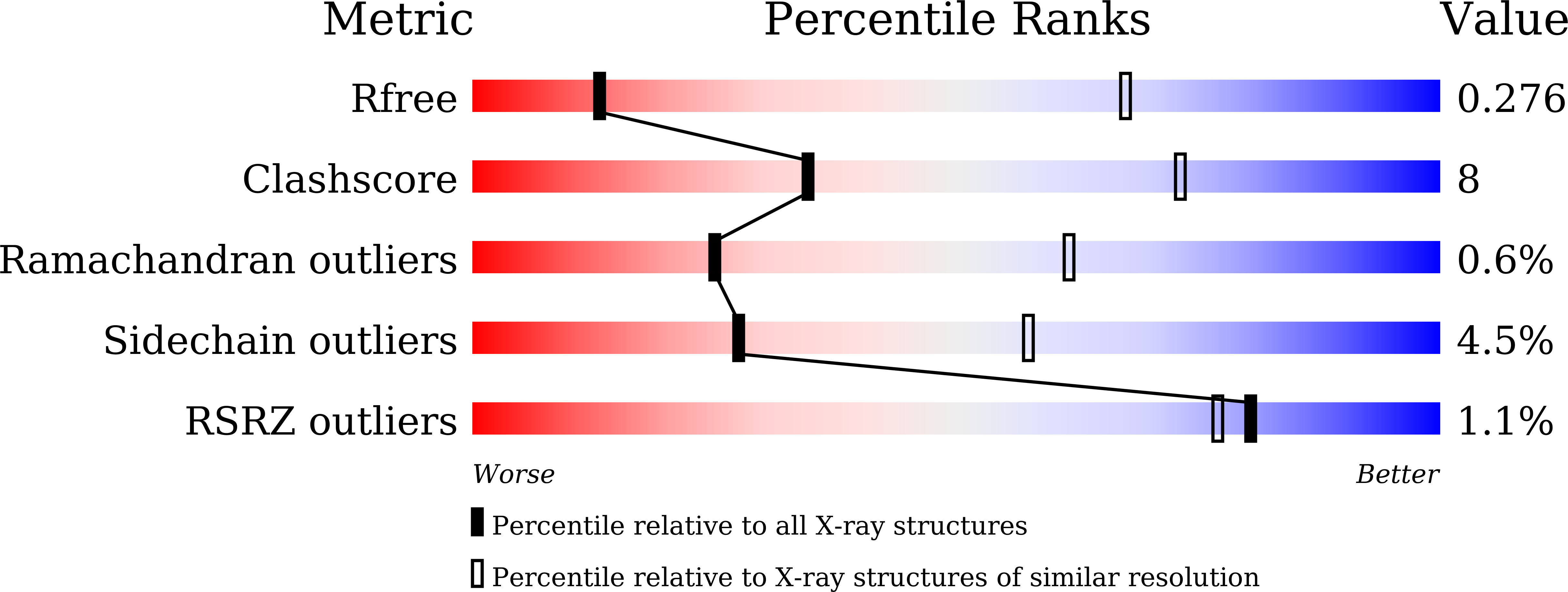
Structural studies on 10-hydroxygeraniol dehydrogenase: A novel linear substrate-specific dehydrogenase from Catharanthus roseus.
Sandholu, A.S., Mujawar, S.P., Ramakrishnan, K., Thulasiram, H.V., Kulkarni, K.(2020) Proteins 88: 1197-1206
- PubMed: 32181958
- DOI: https://doi.org/10.1002/prot.25891
- Primary Citation of Related Structures:
6K3G, 6KJ5 - PubMed Abstract:
Conversion of 10-hydroxygeraniol to 10-oxogeranial is a crucial step in iridoid biosynthesis. This reaction is catalyzed by a zinc-dependent alcohol dehydrogenase, 10-hydroxygeraniol dehydrogenase, belonging to the family of medium-chain dehydrogenase/reductase (MDR). Here, we report the crystal structures of a novel 10-hydroxygeraniol dehydrogenase from Catharanthus roseus in its apo and nicotinamide adenine dinucleotide phosphate (NADP + ) bound forms. Structural analysis and docking studies reveal how subtle conformational differences of loops L1, L2, L3, and helix α9' at the orifice of the catalytic site confer differential activity of the enzyme toward various substrates, by modulating the binding pocket shape and volume. The present study, first of its kind, provides insights into the structural basis of substrate specificity of MDRs specific to linear substrates. Furthermore, comparison of apo and NADP + bound structures suggests that the enzyme adopts open and closed states to facilitate cofactor binding.
Organizational Affiliation:
Division of Biochemical Sciences, CSIR-National Chemical Laboratory, Pune, Maharashtra, India.