Structural and catalytic analysis of two diverse uridine phosphorylases in Phytophthora capsici.
Yang, C., Li, J., Huang, Z., Zhang, X., Gao, X., Zhu, C., Morris, P.F., Zhang, X.(2020) Sci Rep 10: 9051-9051
- PubMed: 32493959
- DOI: https://doi.org/10.1038/s41598-020-65935-9
- Primary Citation of Related Structures:
6K5G, 6K5H, 6K5K, 6K8P - PubMed Abstract:
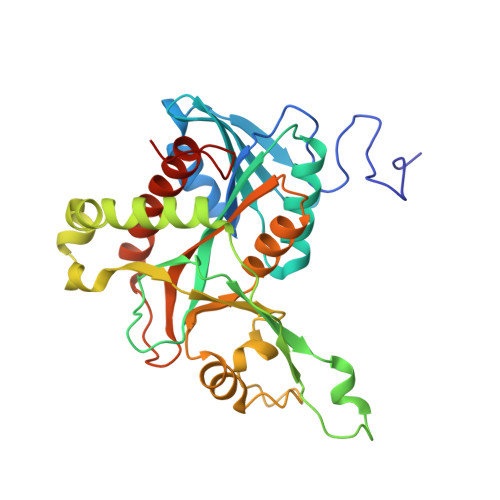
Uridine phosphorylase (UP) is a key enzyme of pyrimidine salvage pathways that enables the recycling of endogenous or exogenous-supplied pyrimidines and plays an important intracellular metabolic role. Here, we biochemically and structurally characterized two evolutionarily divergent uridine phosphorylases, PcUP1 and PcUP2 from the oomycete pathogen Phytophthora capsici. Our analysis of other oomycete genomes revealed that both uridine phosphorylases are present in Phytophthora and Pythium genomes, but only UP2 is seen in Saprolegnia spp. which are basal members of the oomycetes. Moreover, uridine phosphorylases are not found in obligate oomycete pathogens such as Hyaloperonospora arabidopsidis and Albugo spp. PcUP1 and PcUP2 are upregulated 300 and 500 fold respectively, within 90 min after infection of pepper leaves. The crystal structures of PcUP1 in ligand-free and in complex with uracil/ribose-1-phosphate, 2'-deoxyuridine/phosphate and thymidine/phosphate were analyzed. Crystal structure of this uridine phosphorylase showed strict conservation of key residues in the binding pocket. Structure analysis of PcUP1 with bound ligands, and site-directed mutagenesis of key residues provide additional support for the "push-pull" model of catalysis. Our study highlights the importance of pyrimidine salvage during the earliest stages of infection.
Organizational Affiliation:
Shandong Provincial Key Laboratory for Biology of Vegetable Diseases and Insect Pests, College of Plant Protection, Shandong Agricultural University, Tai'an, 271000, China.