Enzymatic synthesis of l-fucose from l-fuculose using a fucose isomerase fromRaoultellasp. and the biochemical and structural analyses of the enzyme.
Kim, I.J., Kim, D.H., Nam, K.H., Kim, K.H.(2019) Biotechnol Biofuels 12: 282-282
- PubMed: 31827610
- DOI: https://doi.org/10.1186/s13068-019-1619-0
- Primary Citation of Related Structures:
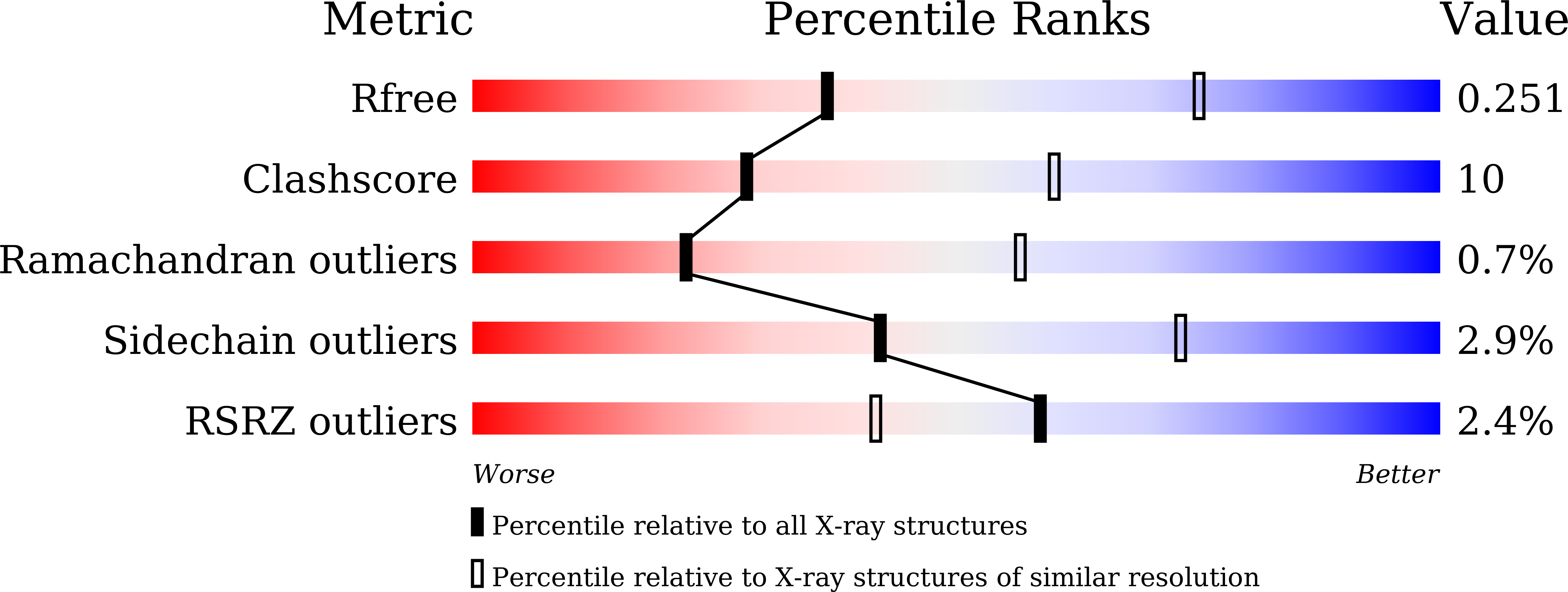
6K1F, 6K1G - PubMed Abstract:
l-Fucose is a rare sugar with potential uses in the pharmaceutical, cosmetic, and food industries. The enzymatic approach using l-fucose isomerase, which interconverts l-fucose and l-fuculose, can be an efficient way of producing l-fucose for industrial applications. Here, we performed biochemical and structural analyses of l-fucose isomerase identified from a novel species of Raoultella ( Rd FucI). Rd FucI exhibited higher enzymatic activity for l-fuculose than for l-fucose, and the rate for the reverse reaction of converting l-fuculose to l-fucose was higher than that for the forward reaction of converting l-fucose to l-fuculose. In the equilibrium mixture, a much higher proportion of l-fucose (~ ninefold) was achieved at 30 °C and pH 7, indicating that the enzyme-catalyzed reaction favors the formation of l-fucose from l-fuculose. When biochemical analysis was conducted using l-fuculose as the substrate, the optimal conditions for Rd FucI activity were determined to be 40 °C and pH 10. However, the equilibrium composition was not affected by reaction temperature in the range of 30 to 50 °C. Furthermore, Rd FucI was found to be a metalloenzyme requiring Mn 2+ as a cofactor. The comparative crystal structural analysis of Rd FucI revealed the distinct conformation of α7-α8 loop of Rd FucI. The loop is present at the entry of the substrate binding pocket and may affect the catalytic activity. Rd FucI-catalyzed isomerization favored the reaction from l-fuculose to l-fucose. The biochemical and structural data of Rd FucI will be helpful for the better understanding of the molecular mechanism of l-FucIs and the industrial production of l-fucose.
Organizational Affiliation:
1Department of Biotechnology, Korea University Graduate School, Seoul, 02841 South Korea.