Structural insights into substrate recognition and catalysis by glycoside hydrolase family 87 alpha-1,3-glucanase from Paenibacillus glycanilyticus FH11.
Itoh, T., Intuy, R., Suyotha, W., Hayashi, J., Yano, S., Makabe, K., Wakayama, M., Hibi, T.(2020) FEBS J 287: 2524-2543
- PubMed: 31788942
- DOI: https://doi.org/10.1111/febs.15161
- Primary Citation of Related Structures:
6K0M, 6K0N, 6K0P, 6K0Q, 6K0S, 6K0U, 6K0V - PubMed Abstract:
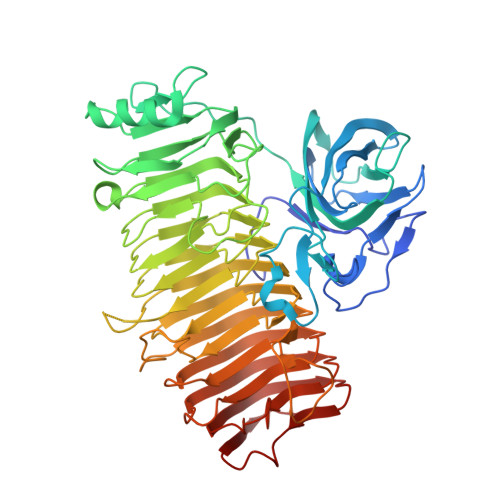
The α-1,3-glucanase from Paenibacillus glycanilyticus FH11 (Agl-FH1), a member of the glycoside hydrolase family 87 (GH87), hydrolyzes α-1,3-glucan with an endo-action. GH87 enzymes are known to degrade dental plaque produced by oral pathogenic Streptococcus species. In this study, the kinetic analyses revealed that this enzyme hydrolyzed α-1,3-tetraglucan into glucose and α-1,3-triglucan with β-configuration at the reducing end by an inverting mechanism. The crystal structures of the catalytic domain (CatAgl-FH1) complexed with or without oligosaccharides at 1.4-2.5 or 1.6 Å resolutions, respectively, are also presented. The initial crystal structure of CatAgl-FH1 was determined by native single-wavelength anomalous diffraction. The catalytic domain was composed of two modules, a β-sandwich fold module, and a right-handed β-helix fold module. The structure of the β-sandwich was similar to those of the carbohydrate-binding module family 35 members. The glycerol or nigerose enzyme complex structures demonstrated that this β-sandwich fold module is a novel carbohydrate-binding module with the capabilities to bind saccharides and to promote the degradation of polysaccharides. The structures of the inactive mutant in complexes with oligosaccharide showed that at least eight subsites for glucose binding were located in the active cleft of the β-helix fold and the architecture of the active cleft was suitable for the recognition and hydrolysis of α-1,3-glucan by the inverting mechanism. The structural similarity to GH28 and GH49 enzymes and the results of site-directed mutagenesis indicated that three Asp residues, Asp1045, Asp1068, and Asp1069, are the most likely candidates for the catalytic residues of Agl-FH1. DATABASE: Structural data are available in RCSB Protein Data Bank under the accession numbers 6K0M (CatAgl-FH1), 6K0N (WT/nigerose), 6K0P (D1045A/nigerose), 6K0Q (D1068A/nigerose), 6K0S (D1069A/ nigerose), 6K0U (D1068A/oligo), and 6K0V (D1069A/oligo). ENZYMES: Agl-FH1, α-1,3-glucanase (EC3.2.1.59) from Paenibacillus glycanilyticus FH11.
Organizational Affiliation:
Department of Bioscience and Biotechnology, Fukui Prefectural University, Eiheiji-cho, Japan.