Fully Productive Cell-Free Genetic Code Expansion by Structure-Based Engineering ofMethanomethylophilus alvusPyrrolysyl-tRNA Synthetase.
Seki, E., Yanagisawa, T., Kuratani, M., Sakamoto, K., Yokoyama, S.(2020) ACS Synth Biol 9: 718-732
- PubMed: 32182048
- DOI: https://doi.org/10.1021/acssynbio.9b00288
- Primary Citation of Related Structures:
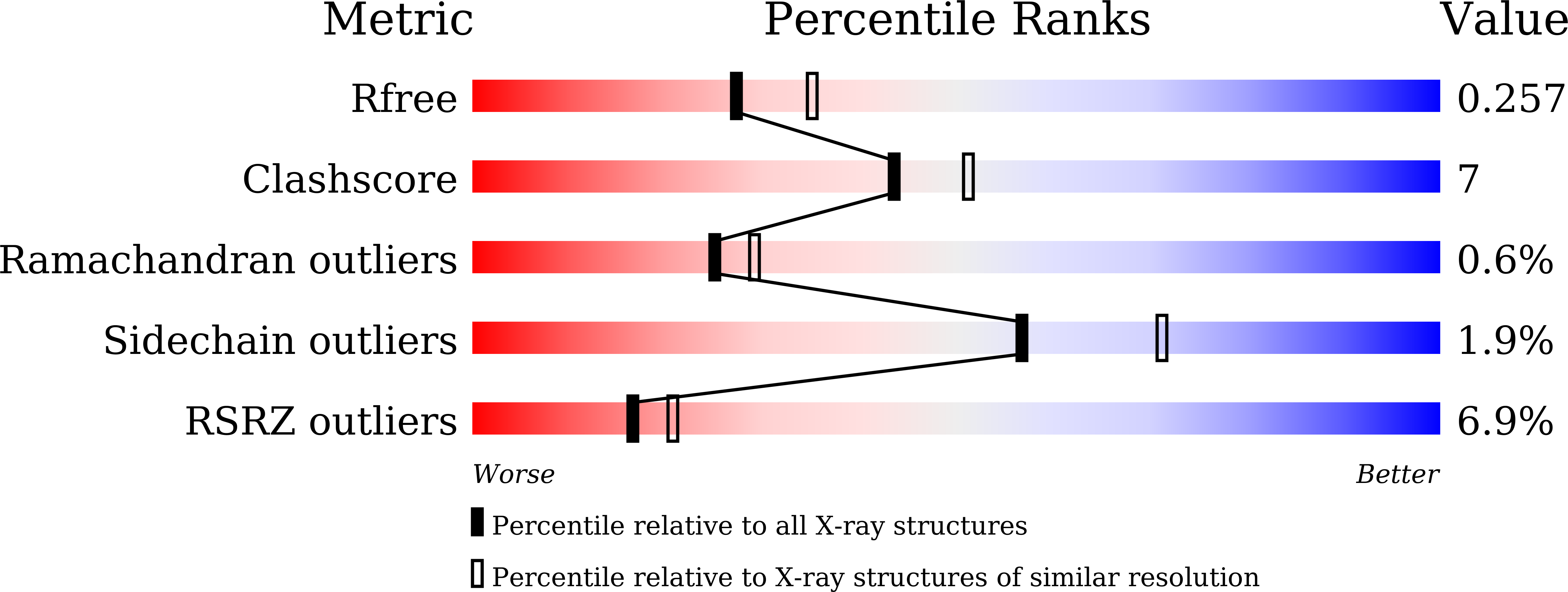
6JP2 - PubMed Abstract:
Pyrrolysyl-tRNA synthetase (PylRS)/tRNA Pyl pairs from Methanosarcina mazei and Methanosarcina barkeri are widely used for site-specific incorporations of non-canonical amino acids into proteins (genetic code expansion). In this study, we achieved the full productivity of cell-free protein synthesis for difficult, bulky non-canonical amino acids, such as N ε -(((( E )-cyclooct-2-en-1-yl)oxy)carbonyl)-l-lysine (TCO*Lys), by using Methanomethylophilus alvus PylRS. First, based on the crystal structure of M. alvus PylRS, the productivities for various non-canonical amino acids were greatly increased by rational engineering of the amino acid-binding pocket. The productivities were further enhanced by using a much higher concentration of PylRS over that of M. mazei PylRS, or by mutating the outer layer of the amino acid-binding pocket. Thus, we achieved full productivity even for TCO*Lys. The quantity and quality of the cell-free-produced antibody fragment containing TCO*Lys were drastically improved. These results demonstrate the importance of full productivity for the expanded genetic code.