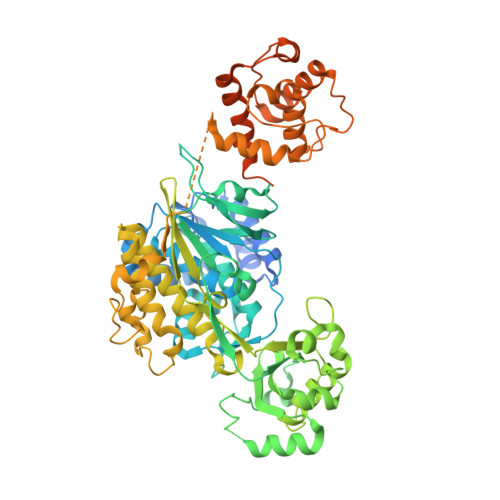
Structural and kinetic insights into flavin-containing monooxygenase and calponin-homology domains in human MICAL3.
Kim, J., Lee, H., Roh, Y.J., Kim, H.U., Shin, D., Kim, S., Son, J., Lee, A., Kim, M., Park, J., Hwang, S.Y., Kim, K., Lee, Y.K., Jung, H.S., Hwang, K.Y., Lee, B.C.(2020) IUCrJ 7: 90-99
- PubMed: 31949908
- DOI: https://doi.org/10.1107/S2052252519015409
- Primary Citation of Related Structures:
6ICI - PubMed Abstract:
MICAL is an oxidoreductase that participates in cytoskeleton reorganization via actin disassembly in the presence of NADPH. Although three MICALs (MICAL1, MICAL2 and MICAL3) have been identified in mammals, only the structure of mouse MICAL1 has been reported. Here, the first crystal structure of human MICAL3, which contains the flavin-containing monooxygenase (FMO) and calponin-homology (CH) domains, is reported. MICAL3 has an FAD/NADP-binding Rossmann-fold domain for mono-oxygenase activity like MICAL1. The FMO and CH domains of both MICAL3 and MICAL1 are highly similar in structure, but superimposition of the two structures shows a different relative position of the CH domain in the asymmetric unit. Based on kinetic analyses, the catalytic efficiency of MICAL3 dramatically increased on adding F-actin only when the CH domain was available. However, this did not occur when two residues, Glu213 and Arg530, were mutated in the FMO and CH domains, respectively. Overall, MICAL3 is structurally highly similar to MICAL1, which suggests that they may adopt the same catalytic mechanism, but the difference in the relative position of the CH domain produces a difference in F-actin substrate specificity.
Organizational Affiliation:
College of Life Sciences and Biotechnology, Korea University, 145 Anam-ro, Seongbuk-gu, Seoul 02841, Republic of Korea.